Dissipative Particle Dynamics Simulation of the Sensitive Anchoring Behavior of Smectic Liquid Crystals at Aqueous Phase
Abstract
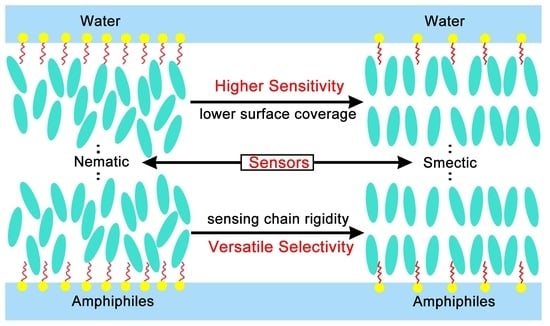
:1. Introduction
2. Simulation Method and Models
2.1. Dissipative Particle Dynamics
2.2. Models
2.3. Simulation Setup
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef]
- Popov, N.; Honaker, L.W.; Popova, M.; Usol’tseva, N.; Mann, E.K.; Jákli, A.; Popov, P. Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials 2018, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Liu, S.; Qi, L.; Ma, C.; Munir, S.; Yu, L.; Hu, Q. Liquid crystal-based sensors for the detection of biomarkers at the aqueous/LC interface. TrAC Trends Anal. Chem. 2021, 144, 116434. [Google Scholar] [CrossRef]
- Oladepo, S.A. Development and Application of Liquid Crystals as Stimuli-Responsive Sensors. Molecules 2022, 27, 1453. [Google Scholar] [CrossRef]
- Gupta, V.K.; Skaife, J.J.; Dubrovsky, T.B.; Abbott, N.L. Optical Amplification of Ligand-Receptor Binding Using Liquid Crystals. Science 1998, 279, 2077–2080. [Google Scholar] [CrossRef] [Green Version]
- Brake, J.M.; Abbott, N.L. An Experimental System for Imaging the Reversible Adsorption of Amphiphiles at Aqueous−Liquid Crystal Interfaces. Langmuir 2002, 18, 6101–6109. [Google Scholar] [CrossRef]
- Brake, J.M.; Mezera, A.D.; Abbott, N.L. Effect of Surfactant Structure on the Orientation of Liquid Crystals at Aqueous−Liquid Crystal Interfaces. Langmuir 2003, 19, 6436–6442. [Google Scholar] [CrossRef]
- Lockwood, N.A.; de Pablo, J.J.; Abbott, N.L. Influence of Surfactant Tail Branching and Organization on the Orientation of Liquid Crystals at Aqueous−Liquid Crystal Interfaces. Langmuir 2005, 21, 6805–6814. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jákli, A. Accurate Optical Detection of Amphiphiles at Liquid-Crystal--Water Interfaces. Phys. Rev. Appl. 2014, 1, 034003. [Google Scholar] [CrossRef]
- Brake Jeffrey, M.; Daschner Maren, K.; Luk, Y.-Y.; Abbott Nicholas, L. Biomolecular Interactions at Phospholipid-Decorated Surfaces of Liquid Crystals. Science 2003, 302, 2094–2097. [Google Scholar] [CrossRef]
- Cumberland, J.; Lopatkina, T.; Murachver, M.; Popov, P.; Kenderesi, V.; Buka, Á.; Mann, E.K.; Jákli, A. Bending nematic liquid crystal membranes with phospholipids. Soft Matter 2018, 14, 7003–7008. [Google Scholar] [CrossRef] [PubMed]
- Kinsinger, M.I.; Buck, M.E.; Meli, M.-V.; Abbott, N.L.; Lynn, D.M. Langmuir films of flexible polymers transferred to aqueous/liquid crystal interfaces induce uniform azimuthal alignment of the liquid crystal. J. Colloid Interface Sci. 2010, 341, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsinger, M.I.; Buck, M.E.; Abbott, N.L.; Lynn, D.M. Immobilization of Polymer-Decorated Liquid Crystal Droplets on Chemically Tailored Surfaces. Langmuir 2010, 26, 10234–10242. [Google Scholar] [CrossRef] [Green Version]
- Hartono, D.; Xue, C.-Y.; Yang, K.-L.; Yung, L.-Y.L. Decorating Liquid Crystal Surfaces with Proteins for Real-Time Detection of Specific Protein–Protein Binding. Adv. Funct. Mater. 2009, 19, 3574–3579. [Google Scholar] [CrossRef]
- Sadati, M.; Apik, A.I.; Armas-Perez, J.C.; Martinez-Gonzalez, J.; Hernandez-Ortiz, J.P.; Abbott, N.L.; de Pablo, J.J. Liquid Crystal Enabled Early Stage Detection of Beta Amyloid Formation on Lipid Monolayers. Adv. Funct. Mater. 2015, 25, 6050–6060. [Google Scholar] [CrossRef]
- Park, C.S.; Iwabata, K.; Sridhar, U.; Tsuei, M.; Singh, K.; Kim, Y.-K.; Thayumanavan, S.; Abbott, N.L. A New Strategy for Reporting Specific Protein Binding Events at Aqueous–Liquid Crystal Interfaces in the Presence of Non-Specific Proteins. ACS Appl. Mater. Interfaces 2020, 12, 7869–7878. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Verma, I.; Pal, S.K. Distinct interfacial ordering of liquid crystals observed by protein–lipid interactions that enabled the label-free sensing of cytoplasmic protein at the liquid crystal-aqueous interface. Analyst 2021, 146, 7152–7159. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, A.R.; Shin, J.-H.; Park, S.-Y. A liquid-crystal-based DNA biosensor for pathogen detection. Sci. Rep. 2016, 6, 22676. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Rather, A.M.; Song, S.; Fang, J.-C.; Dupont, R.L.; Kara, U.I.; Chang, Y.; Paulson, J.A.; Qin, R.; Bao, X.; et al. Ultrasensitive and Selective Detection of SARS-CoV-2 Using Thermotropic Liquid Crystals and Image-Based Machine Learning. Cell Rep. Phys. Sci. 2020, 1, 100276. [Google Scholar] [CrossRef]
- Zafiu, C.; Hussain, Z.; Küpcü, S.; Masutani, A.; Kilickiran, P.; Sinner, E.K. Liquid crystals as optical amplifiers for bacterial detection. Biosens. Bioelectron. 2016, 80, 161–170. [Google Scholar] [CrossRef]
- Sivakumar, S.; Wark, K.L.; Gupta, J.K.; Abbott, N.L.; Caruso, F. Liquid Crystal Emulsions as the Basis of Biological Sensors for the Optical Detection of Bacteria and Viruses. Adv. Funct. Mater. 2009, 19, 2260–2265. [Google Scholar] [CrossRef]
- Lowe, A.M.; Abbott, N.L. Liquid Crystalline Materials for Biological Applications. Chem. Mater. 2012, 24, 746–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani-Dakhel, H.; Rahimi, M.; Pendery, J.; Kim, Y.-K.; Thayumanavan, S.; Roux, B.; Abbott, N.L.; de Pablo, J.J. Amphiphile-Induced Phase Transition of Liquid Crystals at Aqueous Interfaces. ACS Appl. Mater. Interfaces 2018, 10, 37618–37624. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Raghupathi, K.R.; Pendery, J.S.; Khomein, P.; Sridhar, U.; de Pablo, J.J.; Thayumanavan, S.; Abbott, N.L. Oligomers as Triggers for Responsive Liquid Crystals. Langmuir 2018, 34, 10092–10101. [Google Scholar] [CrossRef]
- Iglesias, W.; Abbott, N.L.; Mann, E.K.; Jákli, A. Improving Liquid-Crystal-Based Biosensing in Aqueous Phases. ACS Appl. Mater. Interfaces 2012, 4, 6884–6890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, P. Liquid Crystal Interfaces: Experiments, Simulations and Biosensors. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2015. [Google Scholar]
- Zhang, Z.; Guo, H. A computer simulation study of the anchoring transitions driven by rod–coil amphiphiles at aqueous–liquid crystal interfaces. Soft Matter 2012, 8, 5168–5174. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H.; Nies, E. Mesoscopic simulations of temperature-dependent anchoring and wetting behavior at aqueous–liquid crystal interfaces in the presence of a rod–coil amphiphilic monolayer. RSC Adv. 2018, 8, 42060–42067. [Google Scholar] [CrossRef] [Green Version]
- Español, P.; Warren, P.B. Perspective: Dissipative particle dynamics. J. Chem. Phys. 2017, 146, 150901. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, T.; Nies, E. Mesoscale Simulations of Cylindrical Nanoparticle-Driven Assembly of Diblock Copolymers in Concentrated Solutions. Macromolecules 2014, 47, 5416–5423. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H. The phase behavior, structure, and dynamics of rodlike mesogens with various flexibility using dissipative particle dynamics simulation. J. Chem. Phys. 2010, 133, 144911. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Lockwood, N.A.; Gupta, J.K.; Abbott, N.L. Self-assembly of amphiphiles, polymers and proteins at interfaces between thermotropic liquid crystals and aqueous phases. Surf. Sci. Rep. 2008, 63, 255–293. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, J.; Liu, H.; Qiu, T.; Tang, H.; Zhang, Z. Dissipative Particle Dynamics Simulation of the Sensitive Anchoring Behavior of Smectic Liquid Crystals at Aqueous Phase. Molecules 2022, 27, 7433. https://doi.org/10.3390/molecules27217433
Chen S, Zhang J, Liu H, Qiu T, Tang H, Zhang Z. Dissipative Particle Dynamics Simulation of the Sensitive Anchoring Behavior of Smectic Liquid Crystals at Aqueous Phase. Molecules. 2022; 27(21):7433. https://doi.org/10.3390/molecules27217433
Chicago/Turabian StyleChen, Shiwei, Jinliang Zhang, Huilong Liu, Tongyue Qiu, Haoxiang Tang, and Zunmin Zhang. 2022. "Dissipative Particle Dynamics Simulation of the Sensitive Anchoring Behavior of Smectic Liquid Crystals at Aqueous Phase" Molecules 27, no. 21: 7433. https://doi.org/10.3390/molecules27217433