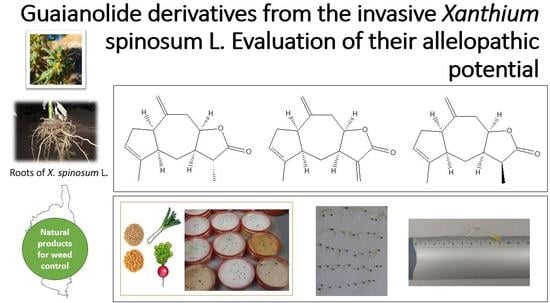
Guaianolide Derivatives from the Invasive Xanthium spinosum L.: Evaluation of Their Allelopathic Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of X. spinosum Root Extracts
2.1.1. Essential Oil and Hydrosol
2.1.2. Hexane Extracts
2.2. Analytical Strategy Applied to Identify X. spinosum Sesquiterpenic Lactones
2.3. Contribution of MS and NMR to the Identification of Lactones from X. spinosum Roots
2.3.1. Ziniolide 66
2.3.2. Xantholide B (11α-dihydroziniolide) 65
2.3.3. 11β-dihydroziniolide 67
2.4. Allelopathic Effect of X. spinosum Root Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Volatile Metabolites
3.3. Fractions
3.4. NaBH4 Reduction
3.5. GC-FID Analysis
3.6. GC-MS Analysis
3.7. NMR Analysis
3.8. Compound Identification and Quantification
11β-dihydroziniolide (67)
3.9. Allelopathic Effect Evaluation
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jeanmonod, D.; Schlüssel, A.; Gamisans, J. Status and Trends in the Alien Flora of Corsica: Status and Trends in the Alien Flora of Corsica. EPPO Bull. 2011, 41, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Blackwell Publishing: Malden, MA, USA, 2007; ISBN 978-1-4051-1418-9. [Google Scholar]
- Máximo, P.; Ferreira, L.M.; Branco, P.S.; Lourenço, A. Invasive Plants: Turning Enemies into Value. Molecules 2020, 25, 3529. [Google Scholar] [CrossRef] [PubMed]
- EPPO Global Database. Available online: https://gd.eppo.int/ (accessed on 5 October 2022).
- Jeanmonod, D.; Gamisans, J. Flora Corsica; Edisud: Aix en Provence, France, 2007; ISBN 978-2-7449-0662-6. [Google Scholar]
- Paradis, G.; Hugot, L.; Spinosi, P. Les Plantes Envahissantes: Une Menace Pour La Biodiversité. Stantari 2008, 13, 18–26. [Google Scholar]
- Weber, E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds; CABI Publishing: Wallingford, UK, 2003; ISBN 978-1-78064-386-1. [Google Scholar]
- Andreani, S.; Paolini, J.; Costa, J.; Muselli, A. Chemical Composition of Essential Oils of Xanthium spinosum L., an Invasive Species of Corsica. Chem. Biodivers. 2017, 14, e1600148. [Google Scholar] [CrossRef]
- Amorin, J.L. Xanthium spinosum L. (Compositae), Weed Used in Argentine Folk Medecine. Rev. Fac. Agron. Univ. Nac. Plata. 1972, 48, 155–169. [Google Scholar]
- Dragendorff, G. Die Heilpflanzen der Verschiedenen Völker und Zeiten: Ihre Anwendung, Wesentlichen Bestandteile und Geschichte; Verlag von Ferdinand Enke: Stuttgart, Germany, 1898. [Google Scholar]
- Varga, E.; Domokos, E.; Kelemen, H.; Fulop, I.; Kursinszki, L. HPLC-ESI-MS/MS Profiling of Phenolic Acids, Flavonoids And Sesquiterpene Lactones from Xanthium spinosum. Rev. Chim. 2020, 71, 558–564. [Google Scholar] [CrossRef]
- Ciocirlan, V. Flora Ilustrata a Romanici; Ceres: Bucuresti, Romania, 2000. [Google Scholar]
- Fernandez, E.; Sandi, Y.; Kokosta, L. Ethnobotanical Inventory of Medicinal Plants Used in the Bustillo Province of the Potosi Department, Bolivia. Fitoterapia 2003, 74, 407–416. [Google Scholar] [CrossRef]
- Hamel, P.B.; Chiltoskey, M.U. Cherokee Plants and Their Uses: A 400 Years History; Herald Publishing: Kings Mountain, NC, USA, 1975; ISBN 978-0-935741-25-4. [Google Scholar]
- Kumar, D.; Kumar, A.; Prakash, O. Potential Antifertility Agents from Plants: A Comprehensive Review. J. Ethnopharmacol. 2012, 140, 1–32. [Google Scholar] [CrossRef]
- Benitez, G.; Gonzalez-Tejero, M.R.; Molero-Mesa, J. Pharmaceuticial Ethnobotany in the Western Part of Granada Province (Southern Spain): Ethnopharmacological Synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Palmese, M.T.; Manganelli, R.E.U.; Tomei, P.E. An Ethno-Pharmacobotanical Survey in the Sarrabus District (South-East Sardinia). Fitoterapia 2001, 72, 619–643. [Google Scholar] [CrossRef]
- Erzsébet, D.; László, K.; Hajnal, K.; Erzsébet, V. A szúrós szerbtövis (Xanthium spinosum L.) növényfaj fitofarmakológiai áttekintése. Acta Pharm. Hung. 2016, 86, 35–40. [Google Scholar]
- Aldibekova, D.A.; Kizaibek, M.; Aisijiang, M.; Dyuskaliyeva, G.; Taldau, A.; Erkinbek, M. Morphology, Anatomy, Chlorogenic Acid Content and Antioxidant Capacity of Xanthium strumarium L. and Xanthium spinosum L. OnLine J. Biol. Sci. 2018, 18, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.H.; Dubey, K.S. 2-Desacetyl-8-Epi-Xanthumanol-4-O-b-D-Galactopyranoside: The Potential Antitumor Sesquiterpenoidal Lactone from Xanthium spinosum Bark. Asian J. Chem. 2000, 12, 521–526. [Google Scholar]
- Yuan, Z.; Zheng, X.; Zhao, Y.; Liu, Y.; Zhou, S.; Wei, C.; Hu, Y.; Shao, H. Phytotoxic Compounds Isolated from Leaves of the Invasive Weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.J.; Terencio, M.C.; Manez, S.; Peris, J.B.; Rios, J.L. Phenolic Compounds from Two Xanthium Species. Planta Med. 1991, 57, A131. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; De Tommasi, N.; De Simone, F. Sesquiterpene and Diterpene Glycosides from Xanthium spinosum. Phytochemistry 1996, 41, 1357–1360. [Google Scholar] [CrossRef]
- Abdei-Mogib, A.; Dawidar, A.M.; Metwally, M.A.; Abou-Elzahab, M. Xanthanolides from Xanthium spinosum. Phytochemistry 1991, 30, 3461–3462. [Google Scholar] [CrossRef]
- Omar, A.A.; Elrashidy, E.M.; Ghazy, N.A.; Metwally, M.A.; Ziesche, J.; Bohlmann, F. Xanthanolides from Xanthium spinosum. Phytochemistry 1984, 23, 915–916. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; Corral, J.; Carda, M.; Jakupovic, J. Xanthanolides from Xanthium: Absolute Configuration of Xanthanol, Isoxanthanol and Their C-4 Epimers. Phytochemistry 1993, 34, 1569–1576. [Google Scholar] [CrossRef]
- Ciulei, I.; Grigorescu, E.; Stanescu, U. Plante Medicinale, Fitochimie, Fitoterapie; Medicala: Bucuresti, Romania, 1993. [Google Scholar]
- Cumanda, J.; Marinoni, G.; Bernardi, M.; Vidari, G.; Finzi, P.V. New Sesquiterpenes from Xanthium Catharticum. J. Nat. Prod. 1991, 54, 460–465. [Google Scholar] [CrossRef]
- Bui, V.-B.; Liu, S.-T.; Zhu, J.-J.; Xiong, J.; Zhao, Y.; Yang, G.-X.; Xia, G.; Hu, J.-F. Sesquiterpene Lactones from the Aerial Parts of Xanthium sibiricum and Their Cytotoxic Effects on Human Cancer Cell Lines. Phytochem. Lett. 2012, 5, 685–689. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Park, S.H.; Choi, S.U.; Lee, C.O.; Kim, S.K.; Kim, Y.K.; Kim, S.H.; Ryu, S.Y. Two Cytotoxic Sesquiterpene Lactone from the Leave of Xanthium strumarium and Their in Vitro Inhibitory Activity of Farnesyltransferase. Planta Med. 2003, 69, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Vasas, A.; Forgo, P.; Rethy, B.; Zupko, I.; Hohmann, J. Xanthanolides with Antitumour Activity from Xanthium Italicum. Z. Nat. C 2009, 64, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Erosa, I.; Huang, Y.; Hickie, R.A.; Sutherland, R.G.; Branka, B. Xanthatin and Xanthinosin from the Burs of Xanthium strumarium L. as Potential Anticancer Agents. Can. J. Physiol. Pharmacol. 2007, 85, 1160–1172. [Google Scholar] [CrossRef]
- Réthy, B.; Csupor-Löffler, B.; Zupko, I.; Hajdu, Z.; Mathé, I.; Hohmann, J.; Rédei, T.; Falkay, G. Antiproliferative Activity of Hungarian Asteraceae Species against Human Cancer Cell Lines. Part I. Phytother. Res. 2007, 21, 1200–1208. [Google Scholar] [CrossRef]
- Little, J.E.; Foote, M.W.; Johnstone, D.B. Xanthatin: An Antimicrobial Agent from Xanthium pennsylvanicum. Arch. Biochem. 1950, 21, 247–254. [Google Scholar]
- Pinel, B.; Audo, G.; Mallet, S.; Lavault, M.; Poype, F.; Seraphin, D.; Richomme, P. Multi Grams Scale Purification of Xanthanolides from Xanthium macrocarpum: Centrifugal Partition Chromatography versus Silica Gel Chromatography. J. Chromatogr. A 2007, 1151, 14–19. [Google Scholar] [CrossRef]
- Tsankova, E.T.; Trendafilova, A.B.; Kujumgiev, A.I.; Galabov, A.S.; Robeva, P.R. Xanthanolides from Xanthium Italicum Moretti and Their Biological Activity. Z. Nat. C 1994, 49, 154–155. [Google Scholar] [CrossRef]
- Ginesta-Peris, E.; Garcia-Breijo, F.J.; Primo-Yúfera, E. Antimicrobial Activity of Xanthatin from Xanthium spinosum L. Lett. Appl. Microbiol. 1994, 18, 206–208. [Google Scholar] [CrossRef]
- Lavault, M.; Landreau, A.; Larcher, G.; Bouchara, J.P.; Pagniez, F.; Le Pape, P.; Richomme, P. Antileschmanial and antifungal activities of xanthanolides isolated from Xanthium macrocarpum. Fitoterapia 2005, 76, 363–366. [Google Scholar] [CrossRef]
- Chandel, S.; Bagai, U.; Vashishat, V. Antiplasmodial Activity of Xanthium strumarium against Plasmodium Berghei-Infected BALB/C Mice. Parasitol. Res. 2012, 110, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Favier, L.S.; Agosta, G.; Gomez, M.R.; Maria, A.G.; Tonn, C.E. Determination and assay validation of the bioactive sesquiterpene lactone from Xanthium cavanillesii usig capillary electrophoresis. Pharmazie 2006, 61, 981–984. [Google Scholar] [PubMed]
- Favier, L.S.; Maria, A.O.M.; Wendel, G.H.; Borkowski, E.J.; Giordano, O.S.; Petzer, L.; Tonn, C.E. Anti-ulcerogenic activity of xanthanolide sesquiterpenes from Xanthium cavanillesii in rats. J. Ethnopharmacol. 2005, 100, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Giner, R.M.; Martini, F.; Schinella, G.R.; Rios, J.L.; Braca, A.; Prieto, J.M. Modulation of COX, LOX and NFκB Activities by Xanthium spinosum L. Root Extract and Ziniolide. Fitoterapia 2013, 91, 284–289. [Google Scholar] [CrossRef]
- Romero, M.; Zanuy, M.; Rosell, E.; Cascante, M.; Piulats, J.; Font-Bardia, M.; Balzarini, J.; De Clerq, E.; Pujol, M.D. Optimization of Xanthatin Extraction from Xanthium spinosum L. and Its Cytotoxic, Anti-Angiogenesis and Antiviral Properties. Eur. J. Med. Chem. 2015, 90, 491–496. [Google Scholar] [CrossRef]
- Linh, N.T.T.; Son, N.T.T.; Ha, N.T.T.; Tra, N.T.; Anh, L.T.T.; Chen, S.; Van Tuyen, N. Biologically Active Constituents from Plants of the Genus Xanthium. Prog. Chem. Org. Nat. Prod. 2021, 116, 135–209. [Google Scholar] [CrossRef]
- Taher, H.A.; Ubiergo, G.O.; Talenti, E.C.J. Constituents of the essential oil of Xanthium cavanillesii. J. Nat. Prod. 1985, 48, 857. [Google Scholar] [CrossRef]
- Sakuda, Y.; Tahara, T. The constituents of essential oil from Xanthium canadense Mill. J. Jpn. Oil Chem. Soc. 1982, 31, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; Li, L.L.; Zeng, L.S.; Zou, F.H.; Feng, S. Study of Volatiles Oils Constituents from 20 Kinds of Compositate in Hunan with GC-MS. J. Univ. Hunan 2010, 30, 28–31. [Google Scholar]
- Habibi, Z.; Laleh, A.; Masoudi, S.; Rustaiyan, A. Composition of the Essential Oil of Xanthium brasilicum Vellozo from Iran. J. Essent. Oil Res. 2004, 16, 31–32. [Google Scholar] [CrossRef]
- Kozhin, S.A.; Ikonnikov, V.V. Composition of essential oil of Xanthium pennsylvanicum Wallroth. Rastit. Resur. 1980, 16, 204–208. [Google Scholar]
- Scherer, R.; Wagner, R.; Meireles, M.A.A.; Godoy, H.T.; Duarte, M.C.T.; Filho, J.T. Biological Activity and Chemical Composition of Hydrodistilled and Supercritical Extracts of Xanthium strumarium L. Leaves. J. Essent. Oil Res. 2010, 22, 424–429. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rustaiyan, A.; Akbari, M.T.; Moazami, N.; Masoudi, S.; Amiri, H. Composition of the Essential Oils of Xanthium strumarium L. and Cetaurea solstitialis L. from Iran. J. Essent. Oil Res. 2006, 18, 427–429. [Google Scholar] [CrossRef]
- Ahuja, M.M.; Nigam, S.S. Chemical examination of the essential oil from the leaves of Xanthium strumarium. Flavour Ind. 1970, 1, 627–630. [Google Scholar]
- El-Gawad, A.; Elshamy, A.; El Gendy, A.; Gaara, A.; Assaeed, A. Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef] [Green Version]
- Andreani, S.; Barboni, T.; Desjobert, J.M.; Paolini, J.; Costa, J.; Muselli, A. Essential Oil Composition and Chemical Variability of Xanthium Italicum Moretti from Corsica. Flavour Fragr. J. 2012, 27, 227–236. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Konig, W.A.; Hochmuth, D.H.; Joulain, D. Terpenoids and Related Constituents of Essential Oils, Library of Mass Finder 2.1; Institute of Organic Chemistry, University of Hamburg: Hamburg, Germany, 2001. [Google Scholar]
- Adams, R.P. Identification of Essential Oils by Capillary Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. Neue Elemanolide Und Guajanolide Aus Zinnia-Arten. Phytochemistry 1979, 18, 1343–1348. [Google Scholar] [CrossRef]
- Kebbi, S.; Ciavatta, M.L.; Mahmoud, A.M.; Carbone, M.; Ligresti, A.; Seghiri, R.; Gavagnin, M. Sesquiterpene Lactones with the 12,8-Guaianolide Skeleton from Algerian Centaurea Omphalotricha. Biomolecules 2021, 11, 1053. [Google Scholar] [CrossRef]
- Tahara, T.; Sakuda, Y. Structure of Xanthanolides A et B, Tow New Guaianolides from Xanthium Canadense Mill. Tetrahedron Lett. 1980, 21, 1861–1862. [Google Scholar] [CrossRef]
- Buděšínský, M.; Šaman, D. Carbon-13 NMR Spectra of Sesquiterpene Lactones. In Annual Reports on NMR Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1995; Volume 30, pp. 231–475. ISBN 978-0-12-505330-3. [Google Scholar]
- Mattson, M.P. Hormesis defined. Ageing Res Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Galindo, J.C.; Massanet, G.M. Potential Allelopathic Activity of Several Sesquiterpene Lactone Models. Phytochemistry 1992, 31, 1969–1977. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive Plants Versus Their New and Old Neighbors: A Mechanism for Exotic Invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Areco, V.A.; Figueroa, S.; Cosa, M.T.; Dambolena, J.S.; Zygadlo, J.A.; Zunino, M.P. Effect of Pinene Isomers on Germination and Growth of Maize. Biochem. Syst. Ecol. 2014, 55, 27–33. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 1997; ISBN 978-92-871-2991-8. [Google Scholar]
- van Der Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments. A Pratical Course. J. Chem. Educ. 1998, 7, 831. [Google Scholar] [CrossRef] [Green Version]
- NIST. Spectral Database for Organic Compounds.; NIST: Gaithersburg, MD, USA, 2008. [Google Scholar]
- NIST. PC Version of the NIST/EPA/NIH Mass Spectral Library; NIST: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Abdelgaleil, S.; Hashinaga, F. Allelopathic Potential of Two Sesquiterpene Lactones from Magnolia grandiflora L. Biochem. Syst. Ecol. 2007, 35, 737–742. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of Germination—Aid In Selection And Evaluation for Seedling Emergence And Vigor. Crop. Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E. Plant Propagation: Principles and Practices; Prentice Hall: Hoboken, NJ, USA, 1983. [Google Scholar]
- van Staden, J.; Sparg, S.G.; Kulkarni, M.G.; Light, M.E. Post-Germination Effects of the Smoke-Derived Compound 3-Methyl-2H-Furo[2,3-c]Pyran-2-One, and Its Potential as a Preconditioning Agent. Field Crop. Res. 2006, 98, 98–105. [Google Scholar] [CrossRef]
- Pannequin, A.; Tintaru, A.; Desjobert, J.-M.; Costa, J.; Muselli, A. New Advances in the Volatile Metabolites of Frullania tamarisci. Flavour Fragr. J. 2017, 32, 409–418. [Google Scholar] [CrossRef]






| N° a | Constituents | RInlit b | RInexp c | RIpexp d | Content (%) e | Identification f | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EO | HYD | MAC | MAE | ||||||||
| 1 | Hexanal | 801 | 770 | 1055 | - | 0.1 | - | - | RI, MS | ||
| 2 | Benzaldehyde | 941 | 929 | 1525 | - | 0.2 | - | - | RI, MS | ||
| 3 | Tricyclene | 927 | 920 | 1020 | 0.1 | - | - | - | RI, MS | ||
| 4 | α-Thujene | 932 | 928 | 1023 | 0.2 | - | - | - | RI, MS | ||
| 5 | α-Pinene | 936 | 931 | 1022 | 1.5 | - | - | - | RI, MS | ||
| 6 | Camphene | 950 | 943 | 1066 | 1.9 | - | - | - | RI, MS | ||
| 7 | β-Pinene | 978 | 970 | 1110 | 1.9 | - | - | - | RI, MS | ||
| 8 | α-Terpinene | 1013 | 1008 | 1178 | 2.0 | - | - | - | RI, MS | ||
| 9 | p-Cymene | 1015 | 1011 | 1268 | 1.1 | - | - | - | RI, MS | ||
| 10 | Limonene | 1025 | 1020 | 1199 | 0.8 | - | - | - | RI, MS | ||
| 11 | γ-Terpinene | 1051 | 1047 | 1243 | 0.6 | - | - | - | RI, MS | ||
| 12 | Terpinolene | 1082 | 1078 | 1280 | 0.2 | - | - | - | RI, MS | ||
| 13 | cis-Sabinene hydrate | 1048 | 1051 | 1605 | - | 0.5 | - | - | RI, MS | ||
| 14 | trans-Sabinene hydrate | 1082 | 1083 | 1541 | - | 0.6 | - | - | RI, MS | ||
| 15 | cis-p-Menth-2-ene-1-ol | 1108 | 1106 | 1621 | - | 0.3 | - | - | RI, MS | ||
| 16 | Camphor | 1123 | 1121 | 1522 | - | 0.1 | - | - | RI, MS | ||
| 17 | trans-p-Menth-2-ene-1-ol | 1123 | 1122 | 1606 | - | 0.2 | - | - | RI, MS | ||
| 18 | trans-Verbenol | 1140 | 1129 | 1676 | - | 0.1 | - | - | RI, MS | ||
| 19 | Mentha-1,5-dien-8-ol | 1166 | 1145 | 1698 | - | 0.1 | - | - | RI, MS | ||
| 20 | Borneol | 1150 | 1148 | 1698 | - | 1.6 | - | - | RI, MS | ||
| 21 | Terpinen-4-ol | 1164 | 1161 | 1600 | 1,0 | 4.9 | - | - | RI, MS | ||
| 22 | α-Terpineol | 1176 | 1179 | 1700 | tr | 0.6 | - | - | RI, MS | ||
| 23 | Cosmen-2-ol | 1187 | 1198 | 1824 | - | 3.9 | - | - | RI, MS | ||
| 24 | Nerol | 1210 | 1211 | 1799 | - | 0.3 | - | - | RI, MS | ||
| 25 | Geraniol | 1235 | 1244 | 1731 | tr | 0.1 | - | - | RI, MS | ||
| 26 | 7α-Silphiperfol-5-ene | 1329 | 1328 | 1429 | - | - | 0.1 | - | RI, MS | ||
| 27 | Silphin-1-ene | 1350 | 1348 | 1474 | 1.2 | - | 0.5 | 0.4 | RI, MS | ||
| 28 | Cyclosativene | 1378 | 1376 | 1483 | 0.2 | - | - | 0.1 | RI, MS | ||
| 29 | Daucene | 1380 | 1382 | 1502 | tr | - | 0.1 | 0.6 | RI, MS | ||
| 30 | α-Copaene | 1379 | 1379 | 1488 | 1.3 | - | 0.3 | 0.5 | RI, MS | ||
| 31 | Modhephene | 1383 | 1382 | 1522 | 1.4 | - | 0.8 | 0.6 | RI, MS | ||
| 32 | α-Isocomene | 1389 | 1388 | 1533 | 6.1 | - | 4.2 | 3.7 | RI, MS | ||
| 33 | β-Elemene | 1389 | 1388 | 1589 | 8.5 | tr | 2.4 | 4.2 | RI, MS | ||
| 34 | β-Isocomene | 1411 | 1406 | 1571 | 2.0 | tr | 1.1 | 0.9 | RI, MS | ||
| 35 | (E)-Caryophyllene | 1421 | 1424 | 1591 | 0.5 | - | 0.5 | 0.5 | RI, MS | ||
| 36 | γ-Elemene | 1429 | 1429 | 1638 | 0.1 | - | 0.2 | - | RI, MS | ||
| 37 | trans-α-Bergamotene | 1434 | 1432 | 1580 | 0.5 | - | 0.4 | 0.4 | RI, MS | ||
| 38 | α-Humulene | 1455 | 1456 | 1665 | 3.6 | tr | 1.6 | 1.2 | RI, MS | ||
| 39 | 4,5-di-epi-Aristocholene | 1471 | 1467 | 1665 | 0.1 | - | - | - | RI, MS | ||
| 40 | γ-Muurolene | 1474 | 1471 | 1681 | - | - | 0.1 | - | RI, MS | ||
| 41 | Germacrene-D | 1479 | 1480 | 1704 | 1.1 | tr | 9.2 | 6.9 | RI, MS | ||
| 42 | β-Selinene | 1486 | 1483 | 1712 | 3.4 | tr | 0.4 | 0.4 | RI, MS | ||
| 43 | α-Selinene | 1494 | 1495 | 1720 | 3.1 | - | 0.4 | 0.5 | RI, MS | ||
| 44 | α-Bulnesene | 1503 | 1502 | 1711 | - | - | - | 0.3 | RI, MS | ||
| 45 | β-Bisabolene | 1503 | 1509 | 1744 | 0.4 | - | 0.4 | 0.3 | RI, MS | ||
| 46 | γ-Cadinene | 1507 | 1507 | 1752 | 0.3 | - | - | 0.6 | RI, MS | ||
| 47 | δ-Cadinene | 1520 | 1516 | 1752 | 1.6 | - | 0.3 | tr | RI, MS | ||
| 48 | trans-Cadina-1,4-diene | 1523 | 1523 | 1763 | 0.2 | - | - | - | RI, MS | ||
| 49 | α-Calacorene | 1527 | 1531 | 1895 | 0.3 | - | - | - | RI, MS | ||
| 50 | β-Calacorene | 1541 | 1548 | 1939 | 0.2 | - | - | - | RI, MS | ||
| 51 | Spathulenol | 1572 | 1568 | 2125 | 0.5 | 0.2 | - | - | RI, MS | ||
| 52 | 4-Formyl-5-nor-β-caryophyllene | 1568 | 1564 | 1994 | - | 0.1 | - | - | RI, MS | ||
| 53 | Neryl 2-methylbutyrate | 1570 | 1565 | 1865 | 6.2 | tr | 2.3 | 1.4 | RI, MS | ||
| 54 | Caryophyllene oxyde | 1578 | 1576 | 1980 | 0.2 | 0.1 | 0.1 | - | RI, MS | ||
| 55 | Carotol | 1594 | 1594 | 2018 | 9.4 | 1.5 | 5.0 | 2.6 | RI, MS | ||
| 56 | Humulene epoxyde II | 1602 | 1601 | 2044 | 1,0 | 0.5 | 0.5 | 0.6 | RI, MS | ||
| 57 | epi-Cubenol | 1623 | 1640 | 2059 | - | 0.3 | - | - | RI, MS | ||
| 58 | α-Cadinol | 1643 | 1645 | 2231 | 1.3 | - | 0.2 | - | RI, MS | ||
| 59 | t-Muurolol | 1633 | 1634 | 2143 | 0.2 | tr | 0.8 | - | RI, MS | ||
| 60 | Selin-11-en-4-α-ol | 1658 | 1659 | 2231 | 3.1 | 2.3 | 0.7 | 3.7 | RI, MS | ||
| 61 | Bulnesol | 1665 | 1659 | 2204 | tr | 0.2 | - | 1.0 | RI, MS | ||
| 62 | 14-Hydroxy-9-epi-(E)-caryophyllene | 1668 | 1657 | 2316 | - | 0.7 | - | - | RI, MS | ||
| 63 | Eudesma-4(15),7-dien-1-β-ol | 1671 | 1672 | 2347 | - | 1.2 | - | - | RI, MS | ||
| 64 | 14-hydroxy-α-Muurolene | 1779 | 1758 | 2531 | 1.1 | 0.9 | 0.3 | - | RI, MS | ||
| 65 | Xantholide B (11-α-dihydroziniolide) | - | 1896 | 2785 | 3.0 | 15.0 | 11.7 | 15.0 | RI, MS, NMR | ||
| 66 | Ziniolide (Xantholide A) | - | 1921 | 2853 | 19.3 | 42.6 | 25.2 | 30.4 | RI, MS, NMR | ||
| 67 | 11-β-dihydroziniolide | - | 1925 | 2838 | tr | 2.1 | 1.8 | - | RI, MS, NMR | ||
| 68 | Hexadecenoic acid | 1951 | 1951 | 2870 | 0.1 | tr | 2.4 | 1.2 | RI, MS | ||
| 69 | Dihydrocollumellarin | 1900 | 1956 | - | 0.9 | - | - | RI, MS | |||
| 70 | Collumellarin | 1952 | 1958 | 2891 | - | 1.0 | - | 6.0 | RI, MS | ||
| Total identified | 92.8 | 83.5 | 74.1 | 84.0 | |||||||
| Hydrocarbon compounds | 46.4 | tr | 23.05 | 22.1 | |||||||
| Oxygenated compounds | 46.4 | 83.5 | 51.0 | 61.9 | |||||||
| Hydrocarbon monoterpenes | 10.3 | - | - | - | |||||||
| Hydrocarbon sesquiterpenes | 36.1 | tr | 23.05 | 22.1 | |||||||
| Oxygenated monoterpenes | 7.2 | 13.3 | 2.3 | 1.4 | |||||||
| Oxygenated sesquiterpenes | 39.1 | 69.9 | 46.3 | 59.3 | |||||||
| Other oxygenated compounds | 0.1 | 0.3 | 2.4 | 1.2 | |||||||
| Sesquiterpenic lactones | 22.3 | 61.6 | 38.7 | 51.4 | |||||||
| C * | 65 δC, type | 66 δC, type | HMBC | 67 δC, type | HMBC |
|---|---|---|---|---|---|
| 1 | 50.10, CH | 51.01, CH | 3, 14 | 50.68, CH | 2, 3, 14 |
| 2 | 35.67, CH2 | 35.96, CH2 | 1, 3 | 35.83, CH2 | 1, 3 |
| 3 | 123.75, CH | 123.98, CH | 1, 2, 15 | 123.84, CH | 2, 15 |
| 4 | 142.55, C | 142.83, C | 2, 15 | 142.70, C | 2, 15 |
| 5 | 51.13, CH | 51.83, CH | 1, 6, 15 | 50.69, CH | 1, 6, 15 |
| 6 | 29.74, CH2 | 31.96, CH2 | 1, 7, 8 | 22.67, CH2 | 1, 11 |
| 7 | 44.00, CH | 42.37, CH | 8, 9, 13 | 43.75, CH | 6, 9, 11 |
| 8 | 79.77, CH | 80.02, CH | 9 | 79.61, CH | 6, 9 |
| 9 | 35.15, CH2 | 34.76, CH2 | 8, 14 | 34.96, CH2 | 14 |
| 10 | 143.68, C | 143.71, C | 1, 9, 14 | 142.56, C | 1, 9, 14 |
| 11 | 45.70, CH | 141.40, C | 7, 13 | 40.57, CH | 6 |
| 12 | 179.70, C | 170.13, C | 13 | 179.03, C | 11, 13 |
| 13 | 15.74, CH3 | 122.15, CH2 | 7 | 10.00, CH3 | 6 |
| 14 | 115.14, CH2 | 115.78, CH2 | 1, 9 | 115.68, CH2 | 1, 9 |
| 15 | 15.04, CH3 | 15.07, CH3 | 15.12, CH3 |
| (a) A. porrum | ||||||||
| Treatment | [C] (µg/mL) | L (mm) * | GR | GP | VI | LR | WR | AE |
| EO | 0 (Control) | 34.2 ± 18.8 a | 2.1 | 86.7 | 3.0 | 1.2 | 0.2 | - |
| 100 | 14.1 ± 10.0 b | 1.8 | 83.3 | 1.2 | 1.1 | 0.2 | −58.8 | |
| 250 | 5.6 ± 6.5 c | 1.2 | 73.3 | 0.4 | 1.3 | 0.2 | −83.5 | |
| 500 | 4.9 ± 4.4 c | 1.1 | 76.7 | 0.4 | 1.3 | 0.2 | −85.8 | |
| 1000 | 3.7 ± 3.2 c | 0.9 | 66.7 | 0.2 | 1.2 | 0.4 | −89.2 | |
| HYD | 0 (Control) | 29.0 ± 20.7 a | 2.2 | 80.0 | 2.3 | 1.2 | 0.2 | - |
| 100 | 15.3 ± 10.7 b | 1.8 | 83.3 | 1.3 | 1.2 | 0.2 | −47.2 | |
| 250 | 7.5 ± 7.4 b,c | 1.2 | 66.7 | 0.5 | 1.1 | 0.1 | −73.4 | |
| 500 | 6.9 ± 6.1 bc | 1.7 | 83.3 | 0.6 | 1.0 | 0.1 | −76.1 | |
| 1000 | 5.3 ± 7.6 c | 0.9 | 60.0 | 0.3 | 1.6 | 0.2 | −81.6 | |
| MAE-F10 | 0 (Control) | 48.6 ± 20.6 a | 2.6 | 93.3 | 4.5 | 1.2 | 0.2 | - |
| 100 | 30.9 ± 21.8 b | 2.2 | 83.3 | 2.6 | 1.3 | 0.2 | −36.4 | |
| 250 | 25.2 ± 14.8 b | 2.0 | 83.3 | 2.1 | 1.4 | 0.1 | −48.2 | |
| 500 | 4.2 ± 7.8 c | 0.9 | 40.0 | 0.2 | 1.9 | 0.3 | −91.3 | |
| 1000 | 2.1 ± 4.5 c | 0.7 | 33.3 | 0.1 | 1.9 | 0.3 | −95.7 | |
| (b) R. sativus | ||||||||
| Treatment | [C] (µg/mL) | L (mm)* | GR | GP | VI | LR | WR | AE |
| EO | 0 (Control) | 147.3 ± 56.2 a | 6.2 | 96.7 | 14.2 | 0.5 | 0.1 | - |
| 100 | 137.6 ± 56.5 a | 6.5 | 96.7 | 13.3 | 0.5 | 0.1 | −6.6 | |
| 250 | 135.5 ± 53.3 a | 6.0 | 96.7 | 13.1 | 0.7 | 0.1 | −8.0 | |
| 500 | 126.4 ± 36.8 a | 5.9 | 100.0 | 12.6 | 0.6 | 0.1 | −14.2 | |
| 1000 | 125.1 ± 37.9 a | 5.5 | 100.0 | 12.5 | 0.6 | 0.1 | −15.1 | |
| HYD | 0 (Control) | 147.3 ± 56.2 a | 6.2 | 96.7 | 14.2 | 0.5 | 0.1 | - |
| 100 | 154.4 ± 45.7 a | 6.0 | 100.0 | 15.4 | 0.5 | 0.1 | 4.9 | |
| 250 | 134.4 ± 51.9 a,b | 5.4 | 100.0 | 13.4 | 0.5 | 0.1 | −8.8 | |
| 500 | 121.7 ± 47.3 b | 4.9 | 96.7 | 11.8 | 0.8 | 0.1 | −17.4 | |
| 1000 | 80.6 ± 37.7 c | 5.3 | 100.0 | 8.1 | 1.0 | 0.1 | −45.3 | |
| MAE-F10 | 0 (Control) | 158.3 ± 50.6 a | 8.5 | 100.0 | 15.8 | 0.4 | 0.1 | - |
| 100 | 152.0 ± 48.7 a,b | 7.9 | 100.0 | 15.2 | 0.4 | 0.1 | −4.0 | |
| 250 | 127.2 ± 57.8 b,c | 8.0 | 100.0 | 12.7 | 0.6 | 0.1 | −19.7 | |
| 500 | 96.1 ± 53.5 c | 7.3 | 100.0 | 9.6 | 0.6 | 0.1 | −39.3 | |
| 1000 | 114.7 ± 39.2 c | 7.3 | 100.0 | 11.5 | 0.6 | 0.1 | −27.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, S.; Bradesi, P.; Muselli, A. Guaianolide Derivatives from the Invasive Xanthium spinosum L.: Evaluation of Their Allelopathic Potential. Molecules 2022, 27, 7297. https://doi.org/10.3390/molecules27217297
Baldi S, Bradesi P, Muselli A. Guaianolide Derivatives from the Invasive Xanthium spinosum L.: Evaluation of Their Allelopathic Potential. Molecules. 2022; 27(21):7297. https://doi.org/10.3390/molecules27217297
Chicago/Turabian StyleBaldi, Sylvain, Pascale Bradesi, and Alain Muselli. 2022. "Guaianolide Derivatives from the Invasive Xanthium spinosum L.: Evaluation of Their Allelopathic Potential" Molecules 27, no. 21: 7297. https://doi.org/10.3390/molecules27217297