An Optimized HPLC-DAD Methodology for the Determination of Anthocyanins in Grape Skins of Red Greek Winegrape Cultivars (Vitis vinifera L.)
Abstract
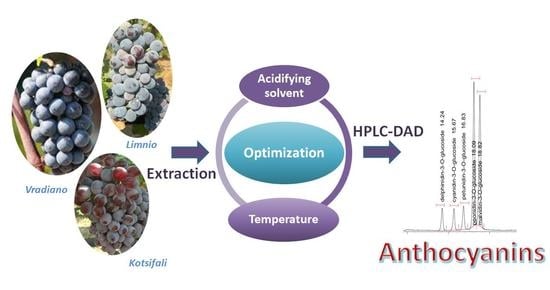
:1. Introduction
2. Results and Discussion
2.1. Optimization of Extraction Parameters
2.1.1. Acidifying Solvent
2.1.2. Extraction Temperature
2.2. Method Validation Results
2.3. Grape Sample Analysis
2.3.1. Identification of Anthocyanins
2.3.2. Quantification Results
2.4. Hierarchical Cluster Analysis
3. Materials and Methods
3.1. Samples Collection
3.2. Sample Preparation
3.3. Reagents and Standards
3.4. Instrumentation
3.5. Extraction of Anthocyanins and Anthocyanidins
3.6. HPLC-DAD Analysis
3.7. Method Validation
3.8. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramawat, K.G.; Mérillon, J.M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1541–2662. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of Climate, Soil, and Cultivar on Terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar]
- Kotseridis, Y.; Georgiadou, A.; Tikos, P.; Kallithraka, S.; Koundouras, S. Effects of Severity of Post-flowering Leaf Removal on Berry Growth and Composition of Three Red Vitis vinifera L. Cultivars Grown under Semiarid Conditions. J. Agric. Food Chem. 2012, 60, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Mohdaly, A.A.-A.; Makris, D.P.; Kefalas, P. Determination of major anthocyanin pigments in Hellenic native grape varieties (Vitis vinifera sp.): Association with antiradical activity. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo Da Silva, J.M. Monomeric, Oligomeric, and Polymeric Flavan-3-ol Composition of Wines and Grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- Mattivi, F.; Scienza, A.; Failla, O.; Villa, P.; Anzani, R.; Tedesco, G.; Gianazza, E.; Righetti, P. Vitis vinifera-a chemotaxonomic approach: Anthocyanins in the skin. In Proceedings of the 5th International Symposium on Grape Breeding, Sankt Martin, Germany, 12–16 September 1989; pp. 119–133. [Google Scholar]
- Liang, Z.; Wu, B.; Fan, P.; Yang, C.; Duan, W.; Zheng, X.; Liu, C.; Li, S. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844. [Google Scholar] [CrossRef]
- Garcia-Muñoz, S.; Muñoz-Organero, G.; De Andrés, M.T.; Cabello, F. Ampelography-An old technique with future uses: The case of minor varieties of Vitis vinifera L. from the Balearic Islands. Oeno One 2011, 45, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gamboa, G.; Liu, S.Y.; Pszczólkowski, P. Resurgence of minority and autochthonous grapevine varieties in South America: A review of their oenological potential. J. Sci. Food Agric. 2020, 100, 465–482. [Google Scholar] [CrossRef]
- Han, F.L.; Xu, Y. Effect of the Structure of Seven Anthocyanins on Self-Association and Colour in an Aqueous Alcohol Solution. S. Afr. J. Enol. Vitic. 2015, 36, 105–116. [Google Scholar] [CrossRef]
- Manzano, S.G.; Santos-Buelga, C.; Dueñas, M.; Rivas-Gonzalo, J.-C.; Escribano-Bailón, T. Colour implications of self-association processes of wine anthocyanins. Eur. Food Res. Technol. 2008, 226, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Castellarin, S.D.; Di Gaspero, G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 7, 46. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhou, Y.; Feng, X. Anthocyanins in Different Food Matrices: Recent Updates on Extraction, Purification and Analysis Techniques. Crit. Rev. Anal. Chem. 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Hussain, A.; Naumovski, N.; Ranjha, M.M.A.N.; Ahmad, N.; Karrar, E.; Xu, B.; Ibrahim, S.A. A Narrative Review of Recent Advances in Rapid Assessment of Anthocyanins in Agricultural and Food Products. Front. Nutr. 2022, 9, 901342. [Google Scholar] [CrossRef] [PubMed]
- López-fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Kalogiouri, N.P.; Samanidou, V.F. Liquid chromatographic methods coupled to chemometrics: A short review to present the key workflow for the investigation of wine phenolic composition as it is affected by environmental factors. Environ. Sci. Pollut. Res. 2021, 28, 59150–59164. [Google Scholar] [CrossRef]
- Oancea, S.; Stoia, M.; Coman, D. Effects of Extraction Conditions on Bioactive Anthocyanin Content of Vaccinium Corymbosum in the Perspective of Food Applications. Procedia Eng. 2012, 42, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Chandra Singh, M.; Kelso, C.; Price, W.E.; Probst, Y. Validated liquid chromatography separation methods for identification and quantification of anthocyanins in fruit and vegetables: A systematic review. Food Res. Int. 2020, 138, 109754. [Google Scholar] [CrossRef]
- Kallithraka, S.; Aliaj, L.; Makris, D.P.; Kefalas, P. Anthocyanin profiles of major red grape (Vitis vinifera L.) varieties cultivated in Greece and their relationship with in vitro antioxidant characteristics. Int. J. Food Sci. Technol. 2009, 44, 2385–2393. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Gkanidi, E.; Koundouras, S.; Mannion, D.T.; Kilcawley, K.N. Discrimination of five Greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. J. Food Compos. Anal. 2020, 92, 103547. [Google Scholar] [CrossRef]
- Pinasseau, L.; Vallverdú-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Péros, J.-P.; Ageorges, A.; Sommerer, N.; Boulet, J.-C.; et al. Cultivar Diversity of Grape Skin Polyphenol Composition and Changes in Response to Drought Investigated by LC-MS Based Metabolomics. Front. Plant Sci. 2017, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Kyraleou, M.; Koundouras, S.; Kallithraka, S.; Theodorou, N.; Proxenia, N.; Kotseridis, Y. Effect of irrigation regime on anthocyanin content and antioxidant activity of Vitis vinifera L. cv. Syrah grapes under semiarid conditions. J. Sci. Food Agric. 2016, 96, 988–996. [Google Scholar] [CrossRef]
- Koundouras, S.; Kanakis, I.; Drossou, E.; Kallithraka, S.; Kotseridis, Y. Effects of postveraison water regime on the phenolic composition of grapes and wines of cv. Agiorgitiko (Vitis vinifera L.). Oeno One 2013, 47, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Chandra Singh, M.; Probst, Y.; Price, W.E.; Kelso, C. Relative comparisons of extraction methods and solvent composition for Australian blueberry anthocyanins. J. Food Compos. Anal. 2021, 105, 104232. [Google Scholar] [CrossRef]
- Pourcel, L.; Irani, N.G.; Lu, Y.; Riedl, K.; Schwartz, S.; Grotewold, E. The Formation of Anthocyanic Vacuolar Inclusions in Arabidopsis thaliana and Implications for the Sequestration of Anthocyanin Pigments. Mol. Plant 2010, 3, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Czitrom, V. One-Factor-at-a-Time versus Designed Experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar] [CrossRef]
- Paun, N.; Botoran, O.R.; Niculescu, V.-C. Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Appl. Sci. 2022, 12, 936. [Google Scholar] [CrossRef]
- Trikas, E.D.; Papi, R.M.; Kyriakidis, D.A.; Zachariadis, G.A. A Sensitive LC-MS Method for Anthocyanins and Comparison of Byproducts and Equivalent Wine Content. Separations 2016, 3, 18. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Marco, S.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine regions. Oeno One 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Zhao, Q.; Duan, C.-Q.; Wang, J. Anthocyanins Profile of Grape Berries of Vitis amurensis, Its Hybrids and Their Wines. Int. J. Mol. Sci. 2010, 11, 2212–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnous, A.; Makris, D.P.; Kefalas, P. Anthocyanin Composition and Colour Characteristics of Selected Aged Wines Produced in Greece. J. Wine Res. 2002, 13, 23–34. [Google Scholar] [CrossRef]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2020, 307, 125518. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Revilla, E.; Cabello, F. Anthocyanin pattern of several red grape cultivars and wines made from them. Eur. Food Res. Technol. 2002, 215, 32–37. [Google Scholar] [CrossRef]
- Kallithraka, S.; Arvanitoyannis, I.S.; Kefalas, P.; El-Zajouli, A.; Soufleros, E.; Psarra, E. Instrumental and sensory analysis of Greek wines; implementation of principal component analysis (PCA) for classification according to geographical origin. Food Chem. 2001, 73, 501–514. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, The Chemistry of Wine: Stabilization and Treatments, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006; Volume 2, ISBN 9780470010396. [Google Scholar]
- Baiano, A.; Terracone, C. Varietal Differences among the Phenolic Profiles and Antioxidant Activities of Seven Table Grape Cultivars Grown in the South of Italy Based on Chemometrics. J. Agric. Food Chem. 2011, 59, 9815–9826. [Google Scholar] [CrossRef]
- Philippidis, A.; Poulakis, E.; Basalekou, M.; Strataridaki, A.; Kallithraka, S.; Velegrakis, M. Characterization of Greek Wines by Ultraviolet–Visible Absorption Spectroscopy and Statistical Multivariate Methods. Anal. Lett. 2017, 50, 1950–1963. [Google Scholar] [CrossRef]
- Ma, T.-T.; Sun, X.-Y.; Gao, G.-T.; Wang, X.-Y.; Liu, X.-Y.; Du, G.-R.; Zhan, J.-C. Phenolic Characterisation and Antioxidant Capacity of Young Wines Made From Different Grape Varieties Grown in Helanshan Donglu Wine Zone (China). S. Afr. J. Enol. Vitic. 2014, 35, 321–331. [Google Scholar] [CrossRef]
- SANTE/12682/2019; Guidance Document on Analytical Quality Control and Method Validation for Pesticide Residues Analysis in Food and Feed. Safe Food Chain Pesticides Biocides European Commission, 2019; pp. 1–48.
- Koskela, A.K.J.; Anttonen, M.J.; Soininen, T.H.; Saviranta, N.M.M.; Auriola, S.; Julkunen-Tiitto, R.; Karjalainen, R.O. Variation in the Anthocyanin Concentration of Wild Populations of Crowberries (Empetrum nigrum L subsp. hermaphroditum). J. Agric. Food Chem. 2010, 58, 12286–12291. [Google Scholar] [CrossRef] [PubMed]





| Acidifier | %R Dlp | %R Cyn | %R Pt | %R Pn | %R Mlv |
|---|---|---|---|---|---|
| 0.05%TFA | 91.0 ± 1.4 | 91.5 ± 1.0 | 101.5 ± 14.6 | 97.7 ± 4.4 | 99.0 ± 0.2 |
| 0.1%TFA | 91.9 ± 2.0 | 91.4 ± 1.4 | 93.1 ± 1.2 | 99.5 ± 2.1 | 101.1 ± 3.3 |
| 0.05%HCl | 110.8 ± 14.8 | 104.9 ± 12.2 | 111.3 ± 11.7 | 116.2 ± 16.9 | 114.8 ± 13.6 |
| 0.1%HCl | 89.3 ± 5.8 | 87.5 ± 2.7 | 95.4 ± 3.5 | 96.3 ± 0.2 | 98.5 ± 2.8 |
| Temperature | Dlp | Cyn | Pt | Pn | Mlv |
|---|---|---|---|---|---|
| 4 °C | 97.9 ± 1.3 | 101.6 ± 1.0 | 102.6 ± 4.7 | 93.8 ± 4.4 | 99.8 ± 0.2 |
| 30 °C | 90.0 ± 6.6 | 91.6 ± 5.2 | 88.5 ± 7.6 | 96.4 ± 2.2 | 91.7 ± 5.4 |
| 50 °C | 98.6 ± 0.9 | 104.9 ± 3.0 | 99.2 ± 0.6 | 104.0 ± 2.2 | 99.1 ± 0.6 |
| Compound | Calibration Equation | Linear range (mg/kg) | r2 | LOD (mg/kg) | LOQ (mg/kg) |
|---|---|---|---|---|---|
| Dlp | y = 72,031.3x − 8267 | LOQ-20 | 0.996 | 0.12 | 0.40 |
| Cyn | y = 77,893.6 x − 6868 | LOQ-20 | 0.999 | 0.10 | 0.30 |
| Pt | y = 73,634.8x + 2305 | LOQ-20 | 0.999 | 0.18 | 0.60 |
| Pn | y = 68,782.6x + 1053 | LOQ-20 | 0.999 | 0.06 | 0.20 |
| Mlv | y = 55,442x + 1895 | LOQ-20 | 0.999 | 0.10 | 0.30 |
| Compound | Low Concentration (%R, n = 3 × 3) | %RSD | Medium Concentration (%R, n = 3 × 3) | %RSD | High Concentration (%R, n = 3 × 3) | %RSD |
|---|---|---|---|---|---|---|
| Dlp | 114 | 3.9 | 112 | 7.4 | 106 | 4.8 |
| Cyn | 105 | 7.5 | 96.7 | 7.9 | 91.6 | 7.3 |
| Pt | 113 | 6.5 | 111 | 6.5 | 106 | 6.2 |
| Pn | 119 | 6.2 | 118 | 5.8 | 107 | 7.1 |
| Mlv | 118 | 8.4 | 116 | 7.4 | 108 | 5.4 |
| Compound | Low Concentration (%R, n = 3 × 3) | %RSD | Medium Concentration (%R, n = 3 × 3) | %RSD | High Concentration (%R, n = 3 × 3) | %RSD |
|---|---|---|---|---|---|---|
| Dlp | 120 | 7.3 | 114 | 2.4 | 111 | 4.4 |
| Cyn | 114 | 5.0 | 94.7 | 2.3 | 89.9 | 3.8 |
| Pt | 118 | 4.7 | 107 | 3.1 | 110 | 6.1 |
| Pn | 103 | 9.6 | 119 | 4.1 | 123 | 1.4 |
| Mlv | 115 | 4.1 | 120 | 6.8 | 117 | 4.4 |
| Compound | RT | λ max (nm) |
|---|---|---|
| Dlp | 14.3 | 516 |
| Cyn | 15.7 | 510 |
| Pt | 16.8 | 543 |
| Pn | 18.1 | 512 |
| Mlv | 18.8 | 520 |
| Variety | Sample Name | Dlp (mg/100 g) | Cyn (mg/100 g) | Pt (mg/100 g) | Pn (mg/100 g) | Mlv (mg/100 g) | Total (mg/100 g) |
|---|---|---|---|---|---|---|---|
| Kotsifali | A1 | 2.02 ± 0.04 | 2.43 ± 0.07 | 1.80 ± 0.04 | 10.4 ± 0.21 | 12.6 ± 0.23 | 29.3 ± 0.58 |
| A2 | 4.27 ± 0.16 | 5.9 ±0.07 | 2.96 ± 0.11 | 13.5 ± 0.32 | 11.6 ± 0.51 | 38.3 ± 1.17 | |
| A3 | 5.35 ± 0.15 | 10.0 ± 0.11 | 3.54 ± 0.17 | 17.6 ± 0.50 | 12.7 ± 0.66 | 49.2 ± 1.59 | |
| A4 | 2.88 ± 0.14 | 5.55 ± 0.33 | 2.16 ± 0.04 | 10.9 ± 0.13 | 10.2 ± 0.29 | 31.7 ± 0.92 | |
| A5 | 1.42 ± 0.08 | 3.35 ± 0.13 | 1.41 ± 0.07 | 6.91 ± 0.26 | 8.25 ± 0.20 | 21.3 ± 0.74 | |
| A6 | 2.38 ± 0.06 | 2.32 ± 0.11 | 2.12 ± 0.03 | 11.0 ± 0.17 | 12.3 ± 0.26 | 30.1 ± 0.72 | |
| Limnio | B1 | 1.07 ± 0.06 | 0.29 ± 0.01 | 1.39 ± 0.07 | 4.07 ± 0.20 | 23.2 ± 0.89 | 30.0 ± 1.23 |
| B2 | 0.26 ± 0.02 | 0.15 ± 0.01 | 0.33 ± 0.03 | 2.51 ± 0.17 | 7.08 ± 0.40 | 10.3 ± 0.64 | |
| B3 | 0.59 ± 0.03 | 0.30 ± 0.01 | 0.68 ± 0.03 | 4.78 ± 0.11 | 10.8 ± 0.55 | 17.2 ± 0.71 | |
| B4 | 0.74 ± 0.14 | 0.37 ± 0.05 | 0.84 ± 0.14 | 4.44 ± 0.57 | 13.6 ± 1.83 | 20.0 ± 2.70 | |
| B5 | 0.23 ± 0.01 | 0.10 ± 0.01 | 0.29 ± 0.01 | 1.40 ± 0.12 | 6.94 ± 0.18 | 8.97 ± 0.32 | |
| B6 | 1.03 ± 0.04 | 0.96 ± 0.16 | 1.19 ± 0.04 | 7.62 ± 0.62 | 14.9 ± 0.89 | 25.7 ± 1.75 | |
| B7 | 0.31 ± 0.01 | 0.17 ± 0.01 | 0.37 ± 0.01 | 1.47 ± 0.06 | 8.22 ± 0.28 | 10.5 ± 0.36 | |
| Vradiano | C1 | 0.75 ± 0.02 | 0.64 ± 0.02 | 0.76 ± 0.02 | 6.25 ± 0.09 | 8.35 ± 0.17 | 16.7 ± 0.31 |
| C2 | 0.60 ± 0.03 | 0.84 ± 0.02 | 0.66 ± 0.03 | 7.88 ± 0.36 | 6.15 ± 0.25 | 16.1 ± 0.69 | |
| C3 | 1.25 ± 0.16 | 0.96 ± 0.05 | 1.30 ± 0.16 | 8.90 ± 0.21 | 10.1 ± 0.64 | 22.5 ± 1.21 |
| Grape Variety | Sample No. | Geographical Origin | Location |
|---|---|---|---|
| Kotsifali | A1 | Crete | Katw Asites |
| A2 | Crete | Dafnes | |
| A3 | Crete | Alagni-Peza | |
| A4 | Crete | Arxanes | |
| A5 | Attica | Wine Institute | |
| A6 | Macedonia | Greek Genebank | |
| Limnio | B1 | Attica | Wine Institute |
| B2 | Macedonia | Epanomi | |
| B3 | Macedonia | Mount Athos | |
| B4 | Macedonia | Sithonia | |
| B5 | Thrace | Xanthi | |
| B6 | Aegean Sea | Limnos | |
| B7 | Macedonia | Serres | |
| Vradiano | C1 | Attica | Wine Institute |
| C2 | Evia | Istiaia | |
| C3 | Evia | Gialtra |
| Time (Min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 22 | 50 | 50 |
| 32 | 5 | 95 |
| 34 | 5 | 95 |
| 35 | 90 | 10 |
| 40 | 90 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogiouri, N.P.; Karadimou, C.; Avgidou, M.S.; Petsa, E.; Papadakis, E.-N.; Theocharis, S.; Mourtzinos, I.; Menkissoglu-Spiroudi, U.; Koundouras, S. An Optimized HPLC-DAD Methodology for the Determination of Anthocyanins in Grape Skins of Red Greek Winegrape Cultivars (Vitis vinifera L.). Molecules 2022, 27, 7107. https://doi.org/10.3390/molecules27207107
Kalogiouri NP, Karadimou C, Avgidou MS, Petsa E, Papadakis E-N, Theocharis S, Mourtzinos I, Menkissoglu-Spiroudi U, Koundouras S. An Optimized HPLC-DAD Methodology for the Determination of Anthocyanins in Grape Skins of Red Greek Winegrape Cultivars (Vitis vinifera L.). Molecules. 2022; 27(20):7107. https://doi.org/10.3390/molecules27207107
Chicago/Turabian StyleKalogiouri, Natasa P., Christina Karadimou, Mary S. Avgidou, Elissavet Petsa, Emmanouil-Nikolaos Papadakis, Serafeim Theocharis, Ioannis Mourtzinos, Urania Menkissoglu-Spiroudi, and Stefanos Koundouras. 2022. "An Optimized HPLC-DAD Methodology for the Determination of Anthocyanins in Grape Skins of Red Greek Winegrape Cultivars (Vitis vinifera L.)" Molecules 27, no. 20: 7107. https://doi.org/10.3390/molecules27207107