Bioaccessibility of Betalains in Beetroot (Beta vulgaris L.) Juice under Different High-Pressure Techniques
Abstract
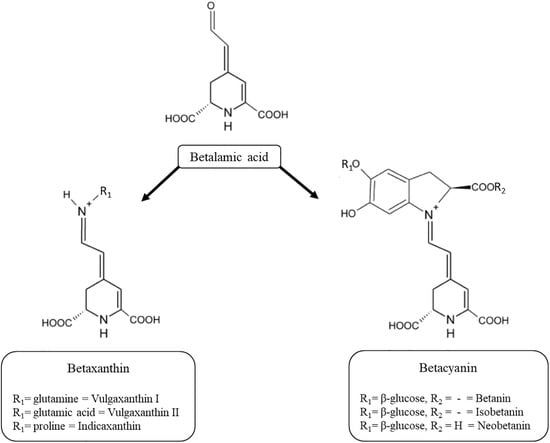
:1. Introduction
2. Results and Discussion
2.1. Effect of HHP and SCCD on Betalains Content in Comparison to Thermal Treatment
2.2. Effect of Simulated Gastrointestinal Digestion on Betalains Content in Beetroot Juice after Different Treatment
2.3. Bioaccessibility of Betalains after HHP and SCCD Treatment in Comparison to Thermal Treatment
2.4. The Influence of Treatment on the Antioxidant Properties of Beetroot Juice in an In Vitro Model of the Gastrointestinal Digestion
3. Materials and Methods
3.1. Reagents and Auxiliary Materials
3.2. Testing Material
3.3. Simulation of Gastrointestinal Digestion and Determination of Bioaccessibility
3.4. Chemical Analysis
3.4.1. Identification of Betalains
3.4.2. HPLC Analyses of Betalains
3.4.3. ABTS+• Radical Assay
3.4.4. DPPH• Radical Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liliana, C.; Oana-Viorela, N. Red Beetroot: Composition and Health Effects—A Review. J. Nutr. Med. Diet Care 2020, 5. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds of Beetroot and Utilization in Food Processing Industry: A Critical Review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Pandita, D.; Pandita, A.; Pamuru, R.R.; Nayik, G.A. Beetroot. In Antioxidants in Vegetables and Nuts—Properties and Health Benefits; Nayik, G.A., Gul, A., Eds.; Springer: Singapore, 2020; pp. 45–74. ISBN 978-981-15-7470-2. [Google Scholar]
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent Developments in Emerging Technologies for Beetroot Pigment Extraction and Its Food Applications. Food Chem. 2021, 356, 129611. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.V.T.; Baião, D.D.S.; Ferreira, V.F.; Paschoalin, V.M.F. Betanin as a Multipath Oxidative Stress and Inflammation Modulator: A Beetroot Pigment with Protective Effects on Cardiovascular Disease Pathogenesis. Crit. Rev. Food Sci. Nutr. 2021, 62, 539–554. [Google Scholar] [CrossRef]
- Lechner, J.F.; Stoner, G.D. Red Beetroot and Betalains as Cancer Chemopreventative Agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Separham, A.; Jafarabadi, M.A. Betalain- and Betacyanin-Rich Supplements’ Impacts on the PBMC SIRT1 and LOX1 Genes Expression and Sirtuin-1 Protein Levels in Coronary Artery Disease Patients: A Pilot Crossover Clinical Trial. J. Funct. Foods 2019, 60, 103401. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jimenez, R.; Michałowski, T.; Wybraniec, S. Influence of Betalain-Rich Extract on Reduction of Discomfortassociated with Osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Sokołowska, B.; Nasiłowska, J. Controlling Spoilage and Pathogenic Microorganisms in Beetroot (Beta vulgaris) Juice by High Hydrostatic Pressure; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128166796. [Google Scholar]
- Marszałek, K.; Szczepańska, J.; Woźniak, Ł.; Skąpska, S.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. The Preservation of Fruit and Vegetable Products under High Pressure Processing. In Encyclopedia of Food Security and Sustainability; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 481–492. ISBN 9780128126875. [Google Scholar]
- Marszałek, K.; Woźniak, Ł.; Kruszewski, B.; Skąpska, S. The Effect of High Pressure Techniques on the Stability of Anthocyanins in Fruit and Vegetables. Int. J. Mol. Sci. 2017, 18, 277. [Google Scholar] [CrossRef] [Green Version]
- Marszałek, K.; Doesburg, P.; Starzonek, S.; Szczepańska, J.; Woźniak, Ł.; Lorenzo, J.M.; Skąpska, S.; Rzoska, S.; Barba, F.J. Comparative Effect of Supercritical Carbon Dioxide and High Pressure Processing on Structural Changes and Activity Loss of Oxidoreductive Enzymes. J. CO2 Util. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Marszałek, K.; Kruszewski, B.; Woźniak, Ł.; Skąpska, S. The Application of Supercritical Carbon Dioxide for the Stabilization of Native and Commercial Polyphenol Oxidases and Peroxidases in Cloudy Apple Juice (Cv. Golden Delicious). Innov. Food Sci. Emerg. Technol. 2017, 39, 42–48. [Google Scholar] [CrossRef]
- Marszałek, K.; Skąpska, S.; Woźniak, Ł.; Sokołowska, B. Application of Supercritical Carbon Dioxide for the Preservation of Strawberry Juice: Microbial and Physicochemical Quality, Enzymatic Activity and the Degradation Kinetics of Anthocyanins during Storage. Innov. Food Sci. Emerg. Technol. 2015, 32, 101–109. [Google Scholar] [CrossRef]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic Modelling of Polyphenol Oxidase, Peroxidase, Pectin Esterase, Polygalacturonase, Degradation of the Main Pigments and Polyphenols in Beetroot Juice during High Pressure Carbon Dioxide Treatment. LWT-Food Sci. Technol. 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional Characteristics of Commercial Beetroot Products and Beetroot Juice Prepared from Seven Beetroot Varieties Grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Klewicka, E. Betacyjaniny—Biodostepność i Biologiczna Aktywność. Food Sci. Technol. Qual. 2012, 19, 5–21. [Google Scholar]
- Wang, T.; Liu, L.; Rakhmanova, A.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Stability of Bioactive Compounds and in Vitro Gastrointestinal Digestion of Red Beetroot Jam: Effect of Processing and Storage. Food Biosci. 2020, 38, 100788. [Google Scholar] [CrossRef]
- Sokołowska, B.; Woźniak, Ł.; Skąpska, S.; Porębska, I.; Nasiłowska, J.; Rzoska, S.J. Evaluation of Quality Changes of Beetroot Juice after High Hydrostatic Pressure Processing. High Press. Res. 2017, 37, 214–222. [Google Scholar] [CrossRef]
- Ubeira-Iglesias, M.; Wilches-Pérez, D.; Cavia, M.M.; Alonso-Torre, S.; Carrillo, C. High Hydrostatic Pressure Processing of Beetroot Juice: Effects on Nutritional, Sensory and Microbiological Quality. High Press. Res. 2019, 39, 691–706. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of Thermal and High Pressure Processing on Quality Parameters of Beetroot (Beta vulgaris L.). LWT-Food Sci. Technol. 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Escobedo-Avellaneda, Z.; Martín-Belloso, O.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; García-García, R.; Torres, J.A.; Welti-Chanes, J. Effect of High Hydrostatic Pressure on the Content of Phytochemical Compounds and Antioxidant Activity of Prickly Pears (Opuntia Ficus-Indica) Beverages. Food Eng. Rev. 2015, 7, 198–208. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Xu, H.; Hao, Q.; Liu, G.; Wang, Q. Inactivation of Peroxidase and Polyphenol Oxidase in Red Beet (Beta vulgaris L.) Extract with Continuous High Pressure Carbon Dioxide. Food Chem. 2010, 119, 108–113. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Peng, X.; Yang, B.; Xu, H.; Zhao, J. Inactivation of Peroxidase and Polyphenol Oxidase in Red Beet (Beta vulgaris L.) Extract with High Pressure Carbon Dioxide. Innov. Food Sci. Emerg. Technol. 2008, 9, 24–31. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Xu, H.; Wang, Q.; Yang, B. Impact of High-Pressure Carbon Dioxide Combined with Thermal Treatment on Degradation of Red Beet (Beta vulgaris L.) Pigments. J. Agric. Food Chem. 2008, 56, 6480–6487. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In Vitro Digestion of Betalainic Foods. Stability and Bioaccessibility of Betaxanthins and Betacyanins and Antioxidative Potential of Food Digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef]
- da Silva, D.V.T.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [Green Version]
- Desseva, I.; Stoyanova, M.; Petkova, N.; Mihaylova, D. Red Beetroot Juice Phytochemicals Bioaccessibility: An in Vitro Approach. Pol. J. Food Nutr. Sci. 2020, 70, 45–53. [Google Scholar] [CrossRef]
- Sawicki, T.; Martinez-Villaluenga, C.; Frias, J.; Wiczkowski, W.; Peñas, E.; Bączek, N.; Zieliński, H. The Effect of Processing and in Vitro Digestion on the Betalain Profile and ACE Inhibition Activity of Red Beetroot Products. J. Funct. Foods 2019, 55, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Maqueo, A.; Steurer, D.; Welti-Chanes, J.; Cano, M.P. Bioaccessibility of Antioxidants in Prickly Pear Fruits Treated with High Hydrostatic Pressure: An Application for Healthier Foods. Molecules 2021, 26, 5252. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Melo, L.M.; Cruz-Cansino, N.D.S.; Delgado-Olivares, L.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Hernández-Traspeña, J.L.; Suárez-Jacobo, Á. Optimization of Antioxidant Activity Properties of a Thermosonicated Beetroot (Beta vulgaris L.) Juice and Further in Vitro Bioaccessibility Comparison with Thermal Treatments. LWT 2022, 154, 112780. [Google Scholar] [CrossRef]
- Stübler, A.S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of Pilot-Scale Processing (Thermal, PEF, HPP) on the Stability and Bioaccessibility of Polyphenols and Proteins in Mixed Protein- and Polyphenol-Rich Juice Systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Trych, U.; Buniowska, M.; Skąpska, S.; Starzonek, S.; Marszałek, K. The Bioaccessibility of Antioxidants in Black Currant Puree after High Hydrostatic Pressure Treatment. Molecules 2020, 25, 3544. [Google Scholar] [CrossRef]
- Trych, U.; Buniowska, M.; Skąpska, S.; Kapusta, I.; Marszałek, K. Bioaccessibility of Antioxidants in Blackcurrant Juice after Treatment Using Supercritical Carbon Dioxide. Molecules 2022, 27, 1036. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Y.; Ma, Y.; Zhao, X. Dense Phase Carbon Dioxide Treatment of Tomato Juice: Effect on Physico-Chemical Properties, Phenolic Composition, Lycopene Isomerisation and in Vitro Bioaccessibility. Int. J. Food Sci. Technol. 2019, 54, 1658–1669. [Google Scholar] [CrossRef]
- Sawicki, T.; Topolska, J.; Bączek, N.; Szawara-Nowak, D.; Juśkiewicz, J.; Wiczkowski, W. Characterization of the Profile and Concentration of Betacyanin in the Gastric Content, Blood and Urine of Rats after an Intragastric Administration of Fermented Red Beet Juice. Food Chem. 2020, 313, 126169. [Google Scholar] [CrossRef]
- Clifford, T.; Constantinou, C.M.; Keane, K.M.; West, D.J.; Howatson, G.; Stevenson, E.J. The Plasma Bioavailability of Nitrate and Betanin from Beta vulgaris Rubra in Humans. Eur. J. Nutr. 2017, 56, 1245–1254. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of Anti-Inflammatory and Antioxidant Activities of Prickly Pear Fruits by High Hydrostatic Pressure: A Chemical and Microstructural Approach. Innov. Food Sci. Emerg. Technol. 2019, 54, 132–142. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of Food Matrix and Processing on the in Vitro Bioaccessibility of Vitamin C, Phenolic Compounds, and Hydrophilic Antioxidant Activity from Fruit Juice-Based Beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of High Hydrostatic Pressure (HHP) on Bioaccessibility, as Well as Antioxidant Activity, Mineral and Starch Contents in Granny Smith Apple. Food Chem. 2011, 128, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the Total Antioxidant Capacity and Total Polyphenol Content of 23 Commercially Available Vegetable Juices before and after in Vitro Digestion Measured by FRAP, DPPH, ABTS and Folin-Ciocalteu Methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Buniowska, M.; Carbonell-Capella, J.M.; Frigola, A.; Esteve, M.J. Bioaccessibility of Bioactive Compounds after Non-Thermal Processing of an Exotic Fruit Juice Blend Sweetened with Stevia Rebaudiana. Food Chem. 2017, 221, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of Processing of Red Beet on Betalain Content and Antioxidant Activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and Nutritional Profiles of Pigment-Enriched Red Beet Root (Beta vulgaris L.) Dried Extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Phytochemistry Plant Betalains: Chemistry and Biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef]
- Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Structural Implications on Color, Fluorescence, and Antiradical Activity in Betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trych, U.; Buniowska-Olejnik, M.; Marszałek, K. Bioaccessibility of Betalains in Beetroot (Beta vulgaris L.) Juice under Different High-Pressure Techniques. Molecules 2022, 27, 7093. https://doi.org/10.3390/molecules27207093
Trych U, Buniowska-Olejnik M, Marszałek K. Bioaccessibility of Betalains in Beetroot (Beta vulgaris L.) Juice under Different High-Pressure Techniques. Molecules. 2022; 27(20):7093. https://doi.org/10.3390/molecules27207093
Chicago/Turabian StyleTrych, Urszula, Magdalena Buniowska-Olejnik, and Krystian Marszałek. 2022. "Bioaccessibility of Betalains in Beetroot (Beta vulgaris L.) Juice under Different High-Pressure Techniques" Molecules 27, no. 20: 7093. https://doi.org/10.3390/molecules27207093