Migration of Microplastics and Phthalates from Face Masks to Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Mask Types and Characterization
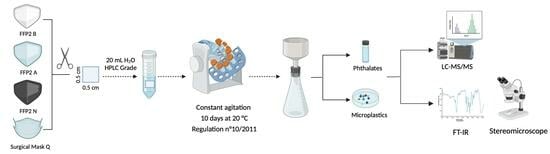
2.3. Migration Experiment
2.4. Microplastics Analysis
2.5. Phthalate Analysis
3. Results and Discussion
3.1. Characterization of Masks
3.2. MP in Masks from Migration Experiment
3.3. Phthalates Released from Masks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
References
- World Health Organization (WHO). Mask Use in the Context of COVID-19; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Fadare, O.O.; Okoffo, E.D. COVID-19 Face Masks: A Potential Source of Microplastic Fibers in the Environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Xu, E.G.; Ren, Z.J. Preventing Masks from Becoming the next Plastic Problem. Front. Environ. Sci. Eng. 2021, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Babaahmadi, V.; Amid, H.; Naeimirad, M.; Ramakrishna, S. Biodegradable and Multifunctional Surgical Face Masks: A Brief Review on Demands during COVID-19 Pandemic, Recent Developments, and Future Perspectives. Sci. Total Environ. 2021, 798, 149233. [Google Scholar] [CrossRef] [PubMed]
- Akber Abbasi, S.; Khalil, A.B.; Arslan, M. Extensive Use of Face Masks during COVID-19 Pandemic: (Micro-)Plastic Pollution and Potential Health Concerns in the Arabian Peninsula. Saudi J. Biol. Sci. 2020, 27, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Saberian, M.; Li, J.; Kilmartin-Lynch, S.; Boroujeni, M. Repurposing of COVID-19 Single-Use Face Masks for Pavements Base/Subbase. Sci. Total Environ. 2021, 769, 145527. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of MP in the Marine Environment: A Global Assessment; Kershaw, P.J., Ed.; International Maritime Organization: London, UK, 2015; Volume 90. [Google Scholar]
- Morgana, S.; Casentini, B.; Amalfitano, S. Uncovering the Release of Micro/Nanoplastics from Disposable Face Masks at Times of COVID-19. J. Hazard. Mater. 2021, 419, 126507. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, F.; Xu, H.; Jiang, H.; Liu, J.; Li, P.; Chen, C.C.; Pan, K. Face Masks as a Source of Nanoplastics and Microplastics in the Environment: Quantification, Characterization, and Potential for Bioaccumulation. Environ. Pollut. 2021, 288, 117748. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Antenucci, S.; Ortenzi, M.A.; Parolini, M. Laundering of Face Masks Represents an Additional Source of Synthetic and Natural Microfibers to Aquatic Ecosystems. Sci. Total Environ. 2022, 806, 150495. [Google Scholar] [CrossRef]
- Xie, H.; Han, W.; Xie, Q.; Xu, T.; Zhu, M.; Chen, J. Face Mask—A Potential Source of Phthalate Exposure for Human. J. Hazard. Mater. 2022, 422, 126848. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Subirats, G.; Rivetti, C.; Cortina-Puig, M.; Barata, C.; Lacorte, S. Occurrence, Toxicity and Risk Assessment of Plastic Additives in Besos River, Spain. Chemosphere 2021, 263, 128022. [Google Scholar] [CrossRef]
- Pérez-Albaladejo, E.; Fernandes, D.; Lacorte, S.; Porte, C. Comparative Toxicity, Oxidative Stress and Endocrine Disruption Potential of Plasticizers in JEG-3 Human Placental Cells. Toxicol. Vitro 2017, 38, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arribas, J.; Moreno, T.; Bartrolí, R.; Eljarrat, E. COVID-19 Face Masks: A New Source of Human and Environmental Exposure to Organophosphate Esters. Environ. Int. 2021, 154, 106654. [Google Scholar] [CrossRef] [PubMed]
- He, M.-J.; Lu, J.-F.; Wang, J.; Wei, S.-Q.; Hageman, K.J. Phthalate Esters in Biota, Air and Water in an Agricultural Area of Western China, with Emphasis on Bioaccumulation and Human Exposure. Sci. Total Environ. 2020, 698, 134264. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.N.; Bush, N.; McElrath, T.F.; Swan, S.H.; Sathyanarayana, S. Joint Impact of Phthalate Exposure and Stressful Life Events in Pregnancy on Preterm Birth. Environ. Int. 2019, 133, 105254. [Google Scholar] [CrossRef] [PubMed]
- Dirinck, E.; Dirtu, A.C.; Geens, T.; Covaci, A.; van Gaal, L.; Jorens, P.G. Urinary Phthalate Metabolites Are Associated with Insulin Resistance in Obese Subjects. Environ. Res. 2015, 137, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human Health Impacts of Exposure to Phthalate Plasticizers: An Overview of Reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, L12, 1–89. [Google Scholar]
- Periyasamy, A.P.; Tehrani-Bagha, A. A Review on Microplastic Emission from Textile Materials and Its Reduction Techniques. Polym. Degrad. Stab. 2022, 199, 109901. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Song, B.; Yi, H.; Hu, T.; Zhang, Y.; Zeng, G.; Xiao, R. Neglected Microplastics Pollution in Global COVID-19: Disposable Surgical Masks. Sci. Total Environ. 2021, 790, 148130. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, X.; Li, Z.; Song, K. COVID-19: Performance Study of Microplastic Inhalation Risk Posed by Wearing Masks. J. Hazard. Mater. 2021, 411, 124955. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Veronelli, M.; Raguso, C.; Barana, D.; Galli, P.; Lasagni, M. The Release Process of Microfibers: From Surgical Face Masks into the Marine Environment. Environ. Adv. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Overview on the Occurrence of Microplastics in Air and Implications from the Use of Face Masks during the COVID-19 Pandemic. Sci. Total Environ. 2021, 800, 149555. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so Colourful: A Potpourri of Plastic Litter Outnumbers Fish Larvae in Europe’s Second Largest River. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics En Route: Field Measurements in the Dutch River Delta and Amsterdam Canals, Wastewater Treatment Plants, North Sea Sediments and Biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S. Disposing of Face Masks: The Next Environmental Problem? Available online: https://www.unicef.org/nepal/stories/disposing-face-masks-next-environmental-problem (accessed on 4 August 2022).
- Beckett, W.S. Occupational Respiratory Diseases. N. Engl. J. Med. 2000, 342, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Liu, Q.; Zhao, Q.; Xiong, X.; Wu, C. Used Disposable Face Masks Are Significant Sources of Microplastics to Environment. Environ. Pollut. 2021, 285, 117485. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, L.; Nack, F.L.; Zimmermann, T.; Pröfrock, D. Microplastics as a Trojan Horse for Trace Metals. J. Hazard. Mater. Lett. 2021, 2, 100035. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2347, Benzyl Butyl Phthalate; National Center for Biotechnology Information: Bethesda, MD, USA, 2012.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6781, Diethyl Phthalate; National Center for Biotechnology Information: Bethesda, MD, USA, 2007.
- Merchant Research & Consulting Ltd. Dioctyl Phthalate (DOP): 2022 World Market Outlook and Forecast up to 2031; Merchant Research & Consulting Ltd.: West Midlands, UK, 2022. [Google Scholar]
- Dionisio, K.L.; Phillips, K.; Price, P.S.; Grulke, C.M.; Williams, A.; Biryol, D.; Hong, T.; Isaacs, K.K. The Chemical and Products Database, a Resource for Exposure-Relevant Data on Chemicals in Consumer Products. Sci. Data 2018, 5, 180125. [Google Scholar] [CrossRef]
- Min, K.; Weng, X.; Long, P.; Ma, M.; Chen, B.; Yao, S. Rapid In-Situ Analysis of Phthalates in Face Masks by Desorption Corona Beam Ionization Tandem Mass Spectrometry. Talanta 2021, 231, 122359. [Google Scholar] [CrossRef] [PubMed]
- Vimalkumar, K.; Zhu, H.; Kannan, K. Widespread Occurrence of Phthalate and Non-Phthalate Plasticizers in Single-Use Facemasks Collected in the United States. Environ. Int. 2022, 158, 106967. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Okoffo, E.D.; Banks, A.P.; Li, Y.; Thomas, K.V.; Rauert, C.; Aylward, L.L.; Mueller, J.F. Phthalate Esters in Face Masks and Associated Inhalation Exposure Risk. J. Hazard. Mater. 2022, 423, 127001. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, J.; Wu, R.; Lin, H.; Lao, J.Y.; Ruan, Y.; Zhang, K.; Wu, J.; Leung, K.M.Y.; Lam, P.K.S. Phthalate Esters in Seawater and Sediment of the Northern South China Sea: Occurrence, Distribution, and Ecological Risks. Sci. Total Environ. 2022, 811, 151412. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EU) 2021/2045 of 23 November 2021 Amending Annex XIV to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off. J. Eur. Union 2021, 418, 6–8. [Google Scholar]
- Aragaw, T.A. Surgical Face Masks as a Potential Source for Microplastic Pollution in the COVID-19 Scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate Quantification and Transport Estimation of Suspended Atmospheric Microplastics in Megacities: Implications for Human Health. Environ. Int. 2019, 132, 105127. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread Distribution of PET and PC Microplastics in Dust in Urban China and Their Estimated Human Exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]














| Type of Mask | Manufacturer | Model | BFE (%) | Regulation | % Library Match with PP | Weight (g) | Area (dm2) |
|---|---|---|---|---|---|---|---|
| FFP2 Blue (FFP2 A) | Chang An Da | CAD–01 | ≥95 | EN 149:2001 + A1:2009 | 95.58 | 4.286 | 2.48 |
| FFP2 White (FFP2 B) | Xiang Ying | XY–9 | ≥94 | EN 149:2001 + A1:2009 | 96.42 | 4.428 | 2.55 |
| FFP2 Black (FPP2 N) | 1 Mi Store | YJ20–02 | ≥94 | EN 149:2001 + A1:2009 | 96.75 | 4.528 | 2.45 |
| Surgical mask (Q) | Starson | N/A | ≥95 | Directive 93/42 | 96.07 | 3.346 | 2.62 |
| Compound | Rt (min) | CV (V) | Q (m/z), CE (eV) | q (m/z), CE (eV) | Linearity | Repeatability (n = 5) | Reprodubility (n = 5) | IDL (μg/mL) | %R± RSD (n = 5) | Bk Levels (μg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| DMP | 3.32 | 15 | 195 → 163 (10) | 195 → 133 (20) | 0.025–1 | 2.72 | 20.5 | 0.00085 | 92 ± 1.2 | ND |
| DEP | 5.05 | 20 | 223 → 149 (20) | 223 → 177 (10) | 0.025–0.75 | 1.69 | 2.99 | 0.00287 | 91 ± 0.6 | 0.007 |
| BBP | 7.80 | 15 | 313 → 91 (10) | 313 → 149 (10) | 0.05–1 | 2.30 | 1.50 | 0.00002 | 101 ± 0.6 | ND |
| DBP | 7.87 | 10 | 279 → 205 (10) | 279 → 121 (10) | 0.05–1 | 1.34 | 2.63 | 0.00035 | 112 ± 0.5 | 2.31 |
| DPP | 8.76 | 35 | 307 → 149 (20) | 307 → 219 (10) | 0.025–0.5 | 3.46 | 2.45 | 0.00006 | 103 ± 0.4 | 0.6 |
| BMPP | 9.15 | 45 | 335 → 167 (10) | 335 → 251 (10) | 0.025–0.75 | 1.47 | 3.09 | 0.00095 | 90 ± 0.4 | 0.29 |
| DNHP | 9.44 | 45 | 335 → 149 (30) | 335 → 121 (40) | 0.05–1 | 1.70 | 2.45 | 0.00029 | 81 ± 1.0 | ND |
| HEHP | 9.95 | 15 | 363 → 149 (20) | 363 → 121 (10) | 0.025–0.75 | 4.47 | 2.19 | 0.00011 | 111 ± 0.8 | ND |
| DEHP | 10.29 | 25 | 391 → 149 (20) | 391 → 167 (10) | 0.25–1 | 1.59 | 1.46 | 0.00007 | 83 ± 11 | 0.001 |
| DNOP | 10.46 | 25 | 391 → 121 (10) | 391 → 261 (10) | 0.025–0.75 | 2.51 | 1.49 | 0.00013 | 97 ± 0.5 | 0.004 |
| DNP | 10.81 | 15 | 419 → 149 (20) | 419 → 275 (10) | 0.05–1 | 1.39 | 3.28 | 0.00003 | 110 ± 1.3 | ND |
| TPhP d15 (IS) | 7.18 | 25 | 342 → 262 (20) | 342 → 161 (30) | na | na | na | na | na | na |
| Sample Name | Fragments | Fibers | ||||
|---|---|---|---|---|---|---|
| PP (%) | PA (%) | Cellulose (%) | PP (%) | PES (%) | Cellulose (%) | |
| FFP2 A | 400 (25) | 400 (25) | 800 (50) | 600 (23) | 200 (8) | 1800 (69) |
| FFP2 B | 200 (25) | 0 | 600 (75) | 400 (10) | 200 (5) | 3600 (85) |
| FFP2 N | 0 | 600 (21) | 2200 (79) | 400 (9) | 1200 (26) | 1500 (65) |
| Surgical Mask Q | 0 | 600 (33) | 1200 (67) | 1000 (20) | 200 (4) | 3800 (76) |
| Blank 1 | 0 | 2 (100) | 0 | 0 | 0 | 7 (100) |
| Blank 2 | 0 | 1 (100) | 0 | 0 | 0 | 6 (100) |
| Blank 3 | 0 | 0 | 0 | 0 | 1 (17) | 5 (83) |
| Sample Name | Fragments | Fibers | |
|---|---|---|---|
| Mean Length (μm) ± SD | Mean Area (μm2) ± SD | Mean Length (μm) ± SD | |
| FFP2 A | 147 ± 69 | 162 ± 76 | 210 ± 156 |
| FFP2 B | 303 ± 226 | 259 ±20 | 339 ± 192 |
| FFP2 N | 113 ± 19 | 115 ± 26 | 358 ± 231 |
| Surgical Mask Q | 209 ± 59 | 183 ±16 | 465 ± 342 |
| Blanks 1 | 114 ± 0.4 | 128 ± 8 | 1217 ± 947 |
| Sample | MP Released per Mask | Type of Study | Study Length | Detection Method | Reference |
|---|---|---|---|---|---|
| FFP2 A | 3968 1 | Migration study in water | 24 h | µ-FT-IR | Present study |
| FFP2 B | 2040 1 | ||||
| FFP2 N | 5390 1 | ||||
| Surgical Mask | 4716 1 | ||||
| N95 | 110 | Breathing simulation | 24 h | Laser Infrared Imaging system | Li et al., 2021 [24] |
| Surgical Mask 1 | 137 | ||||
| Surgical Mask 2 | 264 | ||||
| Cotton | 222 | ||||
| Fashion | 185 | ||||
| Non-woven | 150 | ||||
| Activated carbon | 540 | ||||
| N95 mask 1 | 4400 | ||||
| N95 mask 2 | 3700 | ||||
| Surgical Mask 1 | 1800 | ||||
| Surgical Mask 2 | 1600 | ||||
| Surgical Mask 3 | 1500 | ||||
| Surgical Mask 4 | 1300 | Migration study | 30 min | FT-IR | Ma et al., 2021 [10] |
| Surgical Mask 5 | 1700 | ||||
| Surgical Mask 6 | 1700 | ||||
| Surgical Mask 7 | 1700 | ||||
| Surgical Mask 8 | 2900 | ||||
| Surgical Mask (n = 16) | 28,000 ± 5000 | Shear stress | 120 s | Flow cytometry | Morgana et al., 2021 [9] |
| Surgical Mask 1 | 78,000 | Migration study in seawater | 180 h UV irradiation, 24 h stirring into artificial seawater | µ-FT-IR | Saliu et al., 2021 [25] |
| Surgical Mask 2 | 55,000 | ||||
| Surgical Mask 3 | 173,000 | ||||
| Surgical Mask 4 | 110,000 | ||||
| Surgical Mask 5 | 95,000 | ||||
| Surgical Mask 6 | 67,000 | ||||
| Surgical Mask 7 | 142,000 | ||||
| Surgical Mask reused | 3600 | Migration study in water | 24 h | µ-FT-IR | Shen et al., 2021 [23] |
| Surgical Mask reused | 5400 | Migration study in detergent | |||
| Surgical Mask reused | 4400 | Migration study in alcohol |
| Sample | Phthalate Concentration mg/dm2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DBP | BBP | DNOP | DEHP | DEP | DPP | DMP | Technique | Reference | |
| FFP2 A | 0.0085 | 0.0027 | 0.0020 | 0.0009 | ND | ND | ND | UPLC-MS/MS | Present Study |
| FFP2 B | 0.0025 | 0.0027 | 0.0019 | 0.0018 | ND | ND | ND | ||
| FFP2 N | 0.0045 | 0.0023 | 0.0016 | 0.0011 | ND | ND | ND | ||
| Surgical Mask Q | 0.0010 | 0.0052 | 0.0019 | 0.0016 | ND | ND | ND | ||
| Surgical 1 1 | 1.18 ± 0.05 | 1.72 ± 0.10 | ND | 2.91 ± 0.07 | ND | 0.87 ± 0.02 | ND | in-situ DCBI-MS/MS | Min et al., 2021 [38] |
| Surgical 2 1 | 1.56 ± 0.03 | 1.53 ± 0.05 | ND | 3.74 ± 0.14 | ND | 3.02 ± 0.12 | ND | ||
| Surgical 3 1 | 3.20 ± 0.10 | 2.00 ± 0.09 | ND | 3.29 ± 0.16 | ND | 1.31 ± 0.08 | ND | ||
| N95 1 1 | ND | 1.76 ± 0.09 | ND | 1.28 ± 0.06 | ND | 1.14 ± 0.07 | ND | ||
| N95 2 1 | 0.68 ± 0.04 | 2.76 ± 0.12 | ND | 1.55 ± 0.09 | ND | 0.81 ± 0.04 | ND | ||
| AC 1 1 | ND | 1.93 ± 0.05 | ND | 0.84 ± 0.01 | ND | 2.30 ± 0.06 | ND | ||
| AC 2 1 | ND | 1.77 ± 0.03 | ND | 0.71 ± 0.01 | ND | 1.63 ± 0.05 | ND | ||
| Surgical 1 | - | ND | ND | 0.028 | ND | - | 0.0005 | Pyr-CG/MS | Wang et al., 2022 [40] |
| Surgical 2 | - | ND | ND | 0.020 | 0.0005 | - | 0.0001 | ||
| Surgical 3 | - | ND | ND | ND | ND | - | 0.0005 | ||
| Surgical 4 | - | ND | ND | ND | 0.0027 | - | 0.0004 | ||
| Surgical 5 | - | ND | ND | ND | 0.0016 | - | 0.0003 | ||
| Surgical 6 | - | ND | ND | ND | 0.0077 | - | 0.0001 | ||
| Surgical 7 | - | ND | ND | ND | 0.0062 | - | 0.0001 | ||
| Surgical 8 | - | ND | ND | ND | 0.0011 | - | ND | ||
| Surgical 9 | - | ND | ND | ND | 0.0013 | - | 0.0001 | ||
| Surgical 10 | - | ND | ND | 0.019 | ND | - | 0.0001 | ||
| Surgical 11 | - | ND | ND | ND | 0.0030 | - | 0.0001 | ||
| Surgical 12 | - | ND | ND | ND | ND | - | 0.0003 | ||
| N95 1 | - | ND | ND | 0.057 | 0.0082 | - | ND | ||
| N95 2 | - | ND | ND | 0.12 | 0.0061 | - | 0.0002 | ||
| N95 3 | - | ND | ND | 0.088 | ND | - | 0.0001 | ||
| N95 4 | - | ND | ND | 0.039 | 0.0029 | - | 0.0010 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuri, G.; Oró-Nolla, B.; Torres-Agulló, A.; Karanasiou, A.; Lacorte, S. Migration of Microplastics and Phthalates from Face Masks to Water. Molecules 2022, 27, 6859. https://doi.org/10.3390/molecules27206859
Zuri G, Oró-Nolla B, Torres-Agulló A, Karanasiou A, Lacorte S. Migration of Microplastics and Phthalates from Face Masks to Water. Molecules. 2022; 27(20):6859. https://doi.org/10.3390/molecules27206859
Chicago/Turabian StyleZuri, Giuseppina, Bernat Oró-Nolla, Ana Torres-Agulló, Angeliki Karanasiou, and Silvia Lacorte. 2022. "Migration of Microplastics and Phthalates from Face Masks to Water" Molecules 27, no. 20: 6859. https://doi.org/10.3390/molecules27206859
APA StyleZuri, G., Oró-Nolla, B., Torres-Agulló, A., Karanasiou, A., & Lacorte, S. (2022). Migration of Microplastics and Phthalates from Face Masks to Water. Molecules, 27(20), 6859. https://doi.org/10.3390/molecules27206859