Reversible Sorptive Preconcentration of Noble Metals Followed by FI-ICP-MS Determination
Abstract
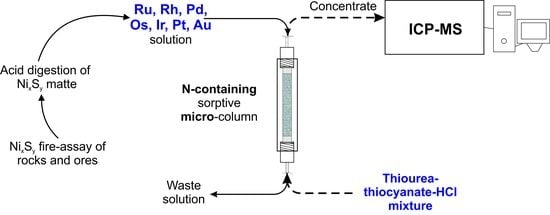
:1. Introduction
2. Results
2.1. Synthesis of PVBC-VP
2.2. FTIR of Blank PVBC-VP and after Sorption of [AuCl4]−
2.3. Sorptive Conditions and Capacity of PVBC-VP
2.4. Conditions of Recovery
2.5. Validation of Full Preconcentration Procedure
3. Discussion
3.1. Influence of PVBC-VP Sorbent Structure on the Sorption of NMs
3.2. Interaction between NM Chlorocomplexes and PVBC-VP
3.3. Conditions of Sorption
3.4. Conditions of Quntitative Recovery
3.5. Validation of the Procedure
4. Materials and Methods
4.1. Reagents
4.2. Sorbent
4.3. Equipment
4.4. Sample Preparation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Komendova, R. Recent advances in the preconcentration and determination of platinum group metals in environmental and biological samples. Trends Anal. Chem. 2020, 122, 115708. [Google Scholar] [CrossRef]
- Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Sanaeepur, H.; Amooghin, A.E.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Danilova, Y.V.; Vasil’eva, I.E.; Shabanova, E.V.; Savelyeva, V.B.; Danilov, B.S. Noble metals in rocks of the sarma group: Phase composition and element associations. Geochem. Int. 2021, 59, 301–313. [Google Scholar] [CrossRef]
- Berezhnaya, E.D.; Dubinin, A.V. Determination of platinum-group elements and gold in ferromanganese nodule reference samples. Geostand. Geoanalytical Res. 2017, 41, 137–145. [Google Scholar] [CrossRef]
- Fontàs, C.; Hidalgo, M.; Salvadó, V. Adsorption and preconcentration of Pd(II), Pt(IV), and Rh(III) using anion-exchange solid-phase extraction cartridges (SPE). Solvent Extr. Ion Exch. 2009, 27, 83–96. [Google Scholar] [CrossRef]
- Myasoedova, G.V.; Antokol’skaya, G.A.; Dmitrieva, S.V.; Kubarev, S.V.; Moiseeva, G.A.; Danilova, F.I.; Grishina, O.N.; Zhukova, N.S.; Savvin, S.B. Sorption of platinum, palladium and rhodium on a chelating sorbent POLYORGS XI and their X-ray fluorescence determination in the concentrate. J. Anal. Chem. 1988, 43, 673–676. [Google Scholar]
- Myasoedova, G.V.; Zaharchenko, E.A.; Mokhodoeva, O.B.; Kubrakova, I.V.; Nikashina, V.A. Sorption Preconcentration of Platinum-Group Metals with Filled Fibrous POLYORGS Sorbents. J. Anal. Chem. 2004, 59, 536–540. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Nabiullina, S.N.; Tyutyunnik, O.A. Au and PGE Determination in Geochemical Materials: Experience in Applying Spectrometric Techniques. Geochem. Int. 2020, 58, 377–390. [Google Scholar] [CrossRef]
- Mokhodoeva, O.B.; Nikulin, A.V.; Myasoedova, G.V.; Kubrakova, I.V. A new combined ETAAS method for the determination of platinum, palladium, and gold traces in natural samples. J. Anal. Chem. 2012, 67, 531–536. [Google Scholar] [CrossRef]
- Park, C.I.; Jeong, J.S.; Cha, G.W. Separation and preconcentration method for palladium, platinum and gold from some heavy metals using Amberlite IRC 718 chelating resin. Bull. Korean Chem. Soc. 2000, 21, 121–124. [Google Scholar] [CrossRef]
- Nagireddi, S.; Golder, A.K.; Uppaluri, R. Role of protonation and functional groups in Pd (II) recovery and reuse characteristics of commercial anion exchange resin-synthetic electroless plating solution systems. J. Water Process Eng. 2018, 22, 227–238. [Google Scholar] [CrossRef]
- Hidalgo, M.; Uheida, A.; Salvadó, V.; Fontàs, C. Study of the sorption and separation abilities of commercial solid-phase extraction (SPE) cartridge oasis MAX towards Au (III), Pd (II), Pt (IV), and Rh (III). Solvent Extr. Ion Exch. 2006, 24, 931–942. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, M.S. Separation of Pt from hydrochloric acid leaching solution of spent catalysts by solvent extraction and ion exchange. Hydrometallurgy 2011, 110, 91–98. [Google Scholar] [CrossRef]
- Yi, Y.V.; Masuda, A. Simultaneous determination of ruthenium, palladium, iridium and platinum at ultratrace levels by isotope dilution inductively coupled plasma mass spectrometry in geological samples. Anal. Chem. 1996, 68, 1444–1450. [Google Scholar] [CrossRef]
- Hashem, M.A.; Elnagar, M.M.; Kenawya, I.M.; Ismail, M.A. Synthesis and application of hydrazono-imidazoline modified cellulose for selective separation of precious metals from geological samples. Carbohydr. Polym. 2020, 237, 116177. [Google Scholar] [CrossRef]
- Karimi, M.; Bazargani, M.F.B.; Aboufazeli, F.; Zhad, H.R.L.; Sadeghi, O.; Najafi, E. Pyridine-functionalized TiO2 nanoparticles as a sorbent for preconcentration and determination of ultra-trace palladium ions. Curr. World Environ. 2012, 7, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Wołowicz, A.; Hubicki, Z. The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Dobrzyńska, J.; Dobrowolski, R.; Olchowski, R.; Zięba, E.; Barczak, M. Palladium adsorption and preconcentration onto thiol-and amine-functionalized mesoporous silicas with respect to analytical applications. Microporous Mesoporous Mater. 2019, 274, 127–137. [Google Scholar] [CrossRef]
- Dal’nova, O.A.; Baranovskaya, V.B.; Dal’nova, Y.S.; Karpov, Y.A. New Complexing Polymer Aminothioether Sorbents in the Analytical Control of Recyclable Metal-Containing Raw Material of Rare and Noble Metals. J. Anal. Chem. 2018, 73, 221–227. [Google Scholar] [CrossRef]
- Losev, V.N.; Parfenova, V.V.; Elsuf’ev, E.V.; Borodina, E.V.; Metelitsa, S.I.; Trofimchuk, A.K. Separation and preconcentration followed by ICP-OES and ICP-MS determination of precious metals using silica gel chemically modified with dithiocarbamate groups. Sep. Sci. Technol. 2020, 55, 2659–2669. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, Y.; Pan, X.; Li, N.; Zhu, J.; Zhu, X. Selenium-doped phenolic resin spheres: Ultra-high adsorption capacity of noble metals. React. Funct. Polym. 2019, 142, 223–230. [Google Scholar] [CrossRef]
- Tyutyunnik, O.A.; Nabiullina, S.N.; Anosova, M.O.; Kubrakova, I.V. Determination of trace amounts of platinum group elements and gold in ultrabasic rocks by inductively coupled plasma mass spectrometry using AG-X8 and LN-Resin sorbents. J. Anal. Chem. 2020, 75, 769–777. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Song, N.; Liu, S.X.; Chen, D.X.; Jia, Q.; Yang, Y.W. Separation and preconcentration of gold and palladium ions with a carboxylated pillar [5] arene derived sorbent prior to their determination by flow injection FAAS. Microchim. Acta 2014, 181, 1551–1556. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Zhang, B. Application of an imidazoline group-containing chelating fiber for the determination of trace noble metals in superhigh-temperature alloys. Fresenius J. Anal. Chem. 2000, 366, 821–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.; Hong, H.J.; Chung, K.W.; Kim, S. Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review. Sep. Purif. Technol. 2020, 246, 116896. [Google Scholar] [CrossRef]
- Dubenskiy, A.S.; Yakurnova, E.D.; Krasilnikova, Y.A.; Seregina, I.F.; Pavlova, L.A.; Davankov, V.A.; Bolshov, M.A. Heterocyclic Amines: New Ion-Pair Reagents for the Simultaneous Reversible Sorption of Noble Metal Chlorocomplexes on a Hyper-Crosslinked Polystyrene Sorbent. Anal. Lett. 2019, 53, 1266–1281. [Google Scholar] [CrossRef]
- Aghaei, E.; Alorro, R.D.; Encila, A.N.; Yoo, K. Magnetic adsorbents for the recovery of precious metals from leach solutions and wastewater. Metals 2017, 7, 529. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, L.A.; Davankov, V.A.; Lependina, O.L. Sorption of complex palladium salts from mineral acid solutions by hypercrosslinked polyvinylpyridinium sorbents (in Russian). Sorbtsionnye I Khromatograficheskie Protsessy 2014, 14, 75–85. [Google Scholar]
- Briones, X.; Tapia, R.A.; Campodónico, P.R.; Urzúa, M.; Leiva, Á.; Contreras, R.; González-Navarrete, J. Synthesis and characterization of poly (ionic liquid) derivatives of N-alkyl quaternized poly (4-vinylpyridine). React. Funct. Polym. 2018, 124, 64–71. [Google Scholar] [CrossRef]
- Katcka, M.; Urbanski, T. Infrared Absorption Spectra of Quaternary Salts of Pyridine. Bull. L’académie Pol. Sci. Ser. Sci. Chim. 1964, 12, 615–621. [Google Scholar]
- Potts, R.A.; Gaj, D.L.; Schneider, W.F.; Dean, N.S.; Kampf, J.W.; Oliver, J.P. Alcoholysis of nitriles in gold (III) complexes: The structure of [EtC(OEt)NH2]+[AuCl4]−. Polyhedron 1991, 10, 1631–1637. [Google Scholar] [CrossRef]
- Usher, A.; McPhail, D.C.; Brugger, J. A spectrophotometric study of aqueous Au (III) halide–hydroxide complexes at 25–80 °C. Geochim. Cosmochim. Acta 2009, 73, 3359–3380. [Google Scholar] [CrossRef]
- Zolotov, Y.A.; Varshal, G.M.; Ivanov, V.M. Analytical Chemistry of Platinum Group Metals (in Russian); Editorial URSS: Moscow, Russia, 2003; p. 592. [Google Scholar]
- Maksimova, Y.A.; Dubenskiy, A.S.; Davankov, V.A.; Pavlova, L.A.; Shigapov, I.V.; Seregina, I.F.; Bolshov, M.A. Conditions and mechanisms of noble metals ions sorption in the process of their preconcentration on the new polyvinylpyridine sorbents. Mon. Für Chem. –Chem. Mon. 2020, 151, 1291–1303. [Google Scholar] [CrossRef]
- Chursanov, Y.V.; Lutsik, V.I.; Starovoytov, S.A.; Potashnikov, Y.M. The kinetics of the cold oxidative dissolution by thiourea-thocyanate system (in Russian). Vestn. Tver. Gos. Univ. Seriya Khimiya 2015, 2, 52–60. [Google Scholar]
- Fedyunina, N.N.; Ossipov, K.B.; Seregina, I.F.; Bolshov, M.A.; Statkus, M.A.; Tsysin, G.I. Determination of rare earth elements in rock samples by inductively coupled plasma mass-spectrometry after sorption preconcentration using Pol-DETATA sorbent. Talanta 2012, 102, 128–131. [Google Scholar] [CrossRef] [PubMed]
- GeoReM: A New Geochemical Database for Reference Materials and Isotopic Standards. Available online: http://georem.mpch-mainz.gwdg.de/sample_query.asp (accessed on 31 July 2022).
- Li, J.; Safarzadeh, M.S.; Moats, M.S.; Miller, J.D.; LeVier, K.M.; Dietrich, M.; Wan, R.Y. Thiocyanate hydrometallurgy for the recovery of gold.: Part II: The leaching kinetics. Hydrometallurgy 2012, 113, 10–18. [Google Scholar] [CrossRef]







| Name of Sorbent | Functional Group | Analytes | Sorptive Capacity, mg g−1 | NM Sorptive Conditions | NM Recovery in Solution | Determination | Issue |
|---|---|---|---|---|---|---|---|
| Modified SBA-15 silica | Thiol- | Pd | 190 | Static mode, >80% in 0.1–1.0 M HNO3 or HCl solutions | 65% recovery by 1.0 M thiourea solution | GFAAS | [19] |
| Amine- | Pd | 68 | Static mode, >80% in 0.05–0.5 M HNO3 or HCl solutions | 100% recovery by 0.6 M thiourea solution | |||
| Imidazoline group- containing chelating fiber | Imidazoline group | Ru, Rh, Pd, Os, Ir, Pt, Au | 69.1 89.8 180.8 194.9 195.9 184.3 724.0 | Dynamic mode in 0.1–1.0 M HCl | Pd, Pt, and Au recovery > 96% by subsequently passing mixture of 1 M HCl and 5% thiourea and then 1 M HClO4 and 5% thiourea. No data about desorption of Ru, Rh, Os, or Ir. | ICP-AES | [25] |
| Aminothioether sorbent | Aminothioether group | Ru, Rh, Pd, Ir, Pt, Au | 300 600 3000 550 1400 3600 | Static mode in 1–3 M HCl | No data, determination in solid concentrate | ETAAS | [20] |
| Chemically modified silica gel | Dithiocarbamate group | Ru, Rh, Pd, Os, Ir, Pt, Au | No data 11.3 * 17 * 11.4 * 11.5 * 13.7 * 7.9 * | Two-column procedure: Au, Pd, and Pt at 20 °C in 0.5–4.0 M HCl and Ru, Rh, Ir, and Os at 95 °C in the presence of 0.025 M SnCl2 | Au, Pd, and Pt were eluted by 10% (w/w) thiourea solution in 1.0 M HCl at 20⁰C and Ir, Ru, Os, and Rh 10% (w/w) thiourea solution in 1.0 M HCl at 95 °C | ICP-AES, ICP-MS | [21] |
| POLYORGS XI | Benzimidazole group | Ru, Rh, Pd, Pt, Au | 108 280 300 297 990 | Static and dynamic mode (8 cm3 wet sorbent) from 2 M HCl | No data, determination in solid concentrate | XRF | [7] |
| POLYORGS XVII | 1,3(5)-Dimethylpyrazol | Rh, Pd, Pt, Au | 58 120 144 180 | Static and dynamic mode, >80% in 0.05–2 M HCl | Digestion of sorbent in HNO3 in a microwave oven | ICP-AES | [8] |
| POLYORGS IV | 3(5)-Methylpyrazole groups | Rh, Pd, Pt, Au | 30 100 100 660 | Static mode (1 h boiling in 1 M HCl) or dynamic mode. | Elution with 2% thiourea solution in 1 M HCl under microwave heating or with acetone at room temperature | ETAAS | [9,10] |
| Amberlite IRC-78 | Iminodiacetic group | Pd, Pt, Au | 58.5 * 60 * 128 * | Static mode, 0.1–4.0 M HCl | >95% recovery by 0.25 M thiourea | ICP-AES | [11,26] |
| Lewatit TP-214 | Thiourea | Pd | 172 | Static mode, pH 8, 300 min | 67% recovery by 0.1 M HCl | AAS | [12] |
| Hydrazono-imidazoline modified cellulose | Hydrazono-imidazoline group | Pd, Pt, Au | 88 105 75 | Static mode, pH 4 | >98% recovery by 0.1 M HNO3 with 0.2 M thiourea | ICP-AES | [16] |
| Pyridine functionalized TiO2 nanoparticles | Pyridine with tertiary nitrogen | Pd | 61 | Dynamic mode, pH 7 | 1 M thiourea in 0.1 M HCl solution | FAAS | [17] |
| Lewatit MonoPlus TP-220 | Pyridine group and tertiary amine group | Pd Pt Au | 10.0 9.9 9.9 | 0.1–6.0 M HCl | Au was quantitatively removed by thiourea and acidic thiourea solutions | AAS | [18] |
| Name of Sorbent | Functional Group | Analytes | Sorptive Capacity, mg g−1 | NM Sorptive Conditions | NM Recovery in Solution | Determination | Issue |
|---|---|---|---|---|---|---|---|
| Dowex 1 × 8(Cl-form) | Quaternary ammonium | Ru, Pd, Ir, Pt, Au | No data | Dynamic mode, 0.5 mL column, 10 mL 0.4 M HCl + Cl2 | Hot (t = 90 °C) 12 M HNO3 recovers > 90% of Pd, Ir, Pt, and Au and ~ 34% of Ru | ICP-MS | [5] |
| Isolute SAX | Quaternary ammonium | Pd, Pt, Rh | 9.6 *, 9.3 *, No data | Dynamic mode, 0.001–1 M HCl | 100% Pt and 100% Pd recovered by 1 M thiourea pH 2. Rh was not recovered. | ICP-AES | [6] |
| Amberlyst A21 | Dimethylamine | Pd | 100 | Static mode, pH 2, 780 min | 55–65% recovery by 0.1–4 M KOH or 0.1–0.5 M thiourea | AAS | [12] |
| Oasis Max | Quaternary ammonium | Rh, Pd, Pt, Au | No data 9.6 12.3 174 * | Dynamic mode, 0.1 M HCl | Pd, Pt, and Au > 95% recovery by 0.5 M thiourea in 1 M HCl | ICP-AES | [13] |
| Bio-Rad AG 1 × 8 | Quaternary ammonium | Ru, Pd, Pt, Ir | No data No data 50 No data | Dynamic mode, HCl solutions | 5 M HCl with 5 M HClO4 | ICP-MS | [14,15] |
| NM Complex | Concentration of HCl in Solution, M | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.20 | 0.40 | 0.60 | 1.00 | 1.25 | 1.50 | 2.00 | 3.00 | 6.00 | |
| [RuCl6]2− | 95 | 96 | 96 | 95 | 94 | 95 | 96 | 95 | 87 | ND * |
| [RhCl6]3− | 97 | 96 | 96 | 94 | 96 | 77 | 78 | 72 | 46 | ND * |
| [PdCl4]2− | 100 | 100 | 100 | 99 | 100 | 100 | 99 | 99 | 99 | ND * |
| [Os(H2O)Cl5]− | 94 | 95 | 95 | 94 | 95 | 100 | 100 | 99 | 100 | ND * |
| [IrCl6]2− | 100 | 100 | 99 | 99 | 99 | 82 | 82 | 75 | 53 | ND * |
| [PtCl4]2− and/or [PtCl6]2− | 100 | 100 | 99 | 100 | 100 | 100 | 100 | 99 | 100 | ND * |
| [AuCl4]− | 99 | 100 | 100 | 99 | 100 | 100 | 100 | 99 | 100 | 99 |
| [Ru2O2(H2O)2Cl6]2− and [Ru2O(H2O)2Cl8]2− and other (1) | [Ru2OCl10]4− (2) | [RuCl6]3− (3) | [RuCl6]2− (4) |
|---|---|---|---|
| 41 ± 3 | 81 ± 4 | 75 ± 5 | 96 ± 3 |
| NMs | Ru | Rh | Pd | Os | Ir | Pt | Au |
|---|---|---|---|---|---|---|---|
| Sorptive capacity, mg g−1 | not below 12.4 | not below 9.1 | 101 | 78 | not below 20 | 140 | 240 |
| NMs | Recovery (R), % | ||
|---|---|---|---|
| 10 mL Tcy-Tu Mixture | Sequential Passing of 5 mL Tu and 5 mL Tcy Collected In One Tube | 10 mL Tu-KCl Mixture | |
| Ru | 86 ± 1 | 68 ± 1 | 36 ± 5 |
| Rh | 77 ± 7 | 57 ± 1 | 33 ± 7 |
| Pd | 92 ± 5 | 79 ± 2 | 70 ± 6 |
| Os | 70 ± 5 | 70 ± 7 | 15 ± 8 |
| Ir | 90 ± 3 | 79 ± 2 | 61 ± 9 |
| Pt | 98 ± 5 | 81 ± 2 | 94 ± 5 |
| Au | 100 ± 3 | 82 ± 4 | 85 ± 4 |
| NMs | Ru | Rh | Pd | Os | Ir | Pt | Au |
|---|---|---|---|---|---|---|---|
| LOD, ng g−1 | 2.2 | 3.7 | 14.7 | 3.4 | 2.4 | 6.5 | 7.8 |
| NMs | SARM-7 | ||
| 1 Certified, ng g−1 | Found, ng g−1 | Found, % | |
| Ru | 430 ± 57 | 400 ± 30 | 93 |
| Rh | 240 ± 13 | 229 ± 17 | 95 |
| Pd | 1530 ± 32 | 1520 ± 80 | 99 |
| Os | 63 ± 4 | 2 22 ± 7 | 35 |
| Ir | 74 ± 12 | 72 ± 14 | 97 |
| Pt | 3740 ± 50 | 3650 ± 70 | 98 |
| Au | 310 ± 15 | 285 ± 10 | 92 |
| NMs | GPt-6 | ||
| 1Certified, ng g−1 | Found, ng g−1 | Found, % | |
| Ru | 13 ± 1 | 12 ± 1 | 97 |
| Rh | 24 ± 3 | 23 ± 4 | 96 |
| Pd | 578 ± 50 | 530 ± 45 | 91 |
| Os | 15.6 ± 0.2 | 2 5 ± 1 | 30 |
| Ir | 28 ± 7 | 32 ± 5 | 91 |
| Pt | 410 ± 40 | 370 ± 30 | 90 |
| Au | 45 ± 10 | 48 ± 8 | 107 |
| NMs | GPt-5 | ||
| 1Certified, ng g−1 | Found, ng g−1 | Found, % | |
| Ru | 528 ± 91 | 584 | 110 |
| Rh | 10 ± 2 | 9.4 ± 2.5 | 94 |
| Pd | 11.3 ± 1.5 | 14.6 | 129 |
| Os | 353 ± 27 | 2 124 | 35 |
| Ir | 136 ± 10 | 140 | 103 |
| Pt | 20 ± 4 | 24 ± 5 | 118 |
| Au | – | 14 ± 4 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimova, Y.A.; Dubenskiy, A.S.; Pavlova, L.A.; Shigapov, I.V.; Korshunov, D.M.; Seregina, I.F.; Davankov, V.A.; Bolshov, M.A. Reversible Sorptive Preconcentration of Noble Metals Followed by FI-ICP-MS Determination. Molecules 2022, 27, 6746. https://doi.org/10.3390/molecules27196746
Maksimova YA, Dubenskiy AS, Pavlova LA, Shigapov IV, Korshunov DM, Seregina IF, Davankov VA, Bolshov MA. Reversible Sorptive Preconcentration of Noble Metals Followed by FI-ICP-MS Determination. Molecules. 2022; 27(19):6746. https://doi.org/10.3390/molecules27196746
Chicago/Turabian StyleMaksimova, Yulia A., Alexander S. Dubenskiy, Lyudmila A. Pavlova, Ilya V. Shigapov, Dmitry M. Korshunov, Irina F. Seregina, Vadim A. Davankov, and Mikhail A. Bolshov. 2022. "Reversible Sorptive Preconcentration of Noble Metals Followed by FI-ICP-MS Determination" Molecules 27, no. 19: 6746. https://doi.org/10.3390/molecules27196746