Semisynthetic Abietic and Dehydroabietic Acid Derivatives and Triptoquinone Epimers Interfere with LPS-Triggered Activation of Dendritic Cells
Abstract
:1. Introduction
2. Results
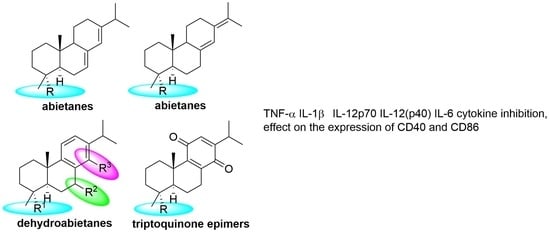
2.1. Screening of 23 Semisynthetic Diterpenes in a DC-Based Bioassay
2.2. Structure–Activity Analysis
2.3. Selected DHA Derivatives and TQs Inhibit DC Maturation in a Dose-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice and Bone Marrow Cell Preparation
4.3. Dendritic Cell (DC) Generation
4.4. Cell Treatments
4.5. Cell Viability, Co-Stimulatory Molecule Expression and Cytokine Secretion
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rashed, A.A.; Rathi, D.-N.G. Bioactive componentes of salvia and their potential antidiabetic properties: A review. Molecules 2021, 26, 3042. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; da Costa, F.B.; de Oliveira, A.M. Diterpenes: A therapeutic promise for cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 1–8. [Google Scholar] [CrossRef]
- Javeed, A.; Ashraf, M.; Riaz, A.; Ghafoor, A.; Afzal, S.; Mukhtar, M.M. Paclitaxel and immune system. Eur. J. Pharm. Sci. 2009, 38, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.B.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.A.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.R.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.V.; Nino-Castro, A.C.; Schultze, J.L. Regulatory dendritic cells: There is more than just immune activation. Front. Immunol. 2012, 3, 274. [Google Scholar] [CrossRef] [Green Version]
- Hackstein, H.; Thomson, A.W. Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 2004, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Correa-Royero, J.; Agudelo, L.; Mesa, A.; Betancur-Galvis, L. Synthesis and biological evaluation of abietic acid derivatives. Eur. J. Med. Chem. 2009, 44, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Pérez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Zapata, B.; Rojas, M.; Betancur-Galvis, L.; Mesa-Arango, A.C.; Pérez-Guaita, D.; González, M.A. Cytotoxic, immunomodulatory, antimycotic, and antiviral activities of semisynthetic 14-hydroxyabietane derivatives and triptoquinone C-4 epimers. Med. Chem. Comm. 2013, 4, 1239–1246. [Google Scholar] [CrossRef]
- Yan, Y.-H.; Shang, P.-Z.; Lu, Q.-J.; Wu, X. Triptolide regulates T cell-mediated immunity via induction of CD11clow dendritic cell differentiation. Food Chem. Toxicol. 2012, 50, 2560–2564. [Google Scholar] [CrossRef]
- Chen, X.; Murakami, T.; Oppenheim, J.J.; Howard, O.M.Z. Triptolide, a constituent of immunosuppressive chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood 2005, 106, 2409–2416. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, Y.; Lamb, J.R.; Tam, P.K.H. Triptolide, a component of chinese herbal medicine, modulates the functional phenotype of dendritic cells. Transplantation 2007, 84, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-J.; Shen, Q.-Y.; Cheng, H.; Mao, X.-H.; Lao, L.-M.; Hao, G.-L. Triptolide affects the differentiation, maturation and function of human dendritic cells. Int. Immunopharmacol. 2005, 5, 1415–1426. [Google Scholar] [CrossRef]
- Montoya Peláez, G.L.; Sierra, J.A.; Alzate, F.; Holzgrabe, U.; Ramirez-Pineda, J.R. Pentacyclic triterpenes from Cecropia telenitida with immunomodulatory activity on dendritic cells. Rev. Bras. Farmacogn. 2013, 23, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Bernal, C.E.; Zorro, M.M.; Sierra, J.; Gilchrist, K.; Botero, J.H.; Baena, A.; Ramirez-Pineda, J.R. Encephalitozoon intestinalis inhibits dendritic cell differentiation through an IL-6-dependent mechanism. Front. Cell. Infect. Microbiol. 2016, 6, 4. [Google Scholar] [CrossRef]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; et al. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 6286. [Google Scholar] [CrossRef] [Green Version]
- Aldahlawi, A.M. Modulation of dendritic cell immune functions by plant components. J. Microsc. Ultrastruct. 2016, 4, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, T. The nuclear factor NF-ΚB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-ΚB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK–STAT Signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Malara, A.; Hambly, R.; Sweeney, C.M.; Kirby, B.; Fletcher, J.M.; Dunne, A. Naturally derived heme-oxygenase 1 inducers attenuate inflammatory responses in human dendritic cells and T cells: Relevance for psoriasis treatment. Sci. Rep. 2018, 8, 10287. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-H.; Son, Y.; Jeong, S.-O.; Moon Hur, J.; Soo Bang, H.; Lee, K.-N.; Kim, E.-C.; Chung, H.-T.; Pae, H.-O. Tetrahydroabietic acid, a reduced abietic acid, inhibits the production of inflammatory mediators in RAW264.7 macrophages activated with lipopolysaccharide. J. Clin. Biochem. Nutr. 2010, 46, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Lee, J.-Y.; Uemura, T.; Ezaki, Y.; Takahashi, N.; Kawada, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Li, Y.-C.; You, B.-J.; Chang, W.-T.; Chao, L.K.; Lo, L.-C.; Wang, S.-Y.; Huang, G.-J.; Kuo, Y.-H. Diterpenoids with anti-inflammatory activity from the wood of Cunninghamia konishii. Molecules 2013, 18, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Kim, J.-R.; Han, J.-M.; Jang, K.C.; Sok, D.-E.; Jeong, T.-S. Antioxidant activities of abietane diterpenoids isolated from Torreya nucifera leaves. J. Agric. Food Chem. 2006, 54, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.-F.; Zhang, G.-J.; Chen, H.; Bai, J.; Yu, S.-S.; Liu, Y.; Wang, W.-J.; Ma, S.-G.; Qu, J.; Xu, S. Diterpenoids and sesquiterpenoids from the twigs and leaves of Illicium majus. Planta Med. 2013, 79, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Yu, T.; Choi, S.J.; Yang, Y.; Baek, H.S.; An, S.A.; Kwon, L.K.; Kim, J.; Rho, H.S.; Shin, S.S.; et al. Syk/Src Pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediators Inflamm. 2012, 2012, 781375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-W.; Kundu, J.; Chae, I.-G.; Kim, D.-H.; Yu, M.-H.; Kundu, J.K.; Chun, K.-S. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int. J. Oncol. 2014, 44, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-H.; Xie, Y.-X.; Zhang, J.-W.; Qiu, X.-H.; Cheng, A.-B.; Tian, L.; Ma, B.-Y.; Hou, Y.-B. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J. Recept. Signal Transduct. 2016, 36, 72–78. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Guha, R. On Exploring Structure–Activity Relationships. Methods Mol. Biol. 2013, 993, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Shishido, K.; Nakano, K.; Wariishi, N.; Tateishi, H.; Omodani, T.; Shibuya, M.; Goto, K.; Ono, Y.; Takaishi, Y. Tripterygium wilfordii var. Regelii which are interleukin-1 inhibitors. Phytochemistry 1994, 35, 731–737. [Google Scholar] [CrossRef]
- Takaishi, Y.; Shishido, K.; Wariishi, N.; Shibuya, M.; Goto, K.; Kido, M.; Taka, M.; Ono, Y. Triptoquinone A and B novel interleukin-1 inhibitors from Tripterygium wilfordii var regeli. Tetrahedron Lett. 1992, 33, 7177–7180. [Google Scholar] [CrossRef]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.; Rößner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]



| Compound | Concentration µM | Cytokine Concentration in ng/mL (% Inhibition) | Cell Viability | ||||
|---|---|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-12p70 | IL-12p40 | IL-6 | |||
| Control | ND | 0.06 ± 0.04 | 0.01 ± 0.013 | 0.27 ± 0.08 | 0.03 ± 0.05 | 100.4 | |
| Vehicle | 0.01 ± 0.01 | 0.08 ± 0.06 | 0.01 ± 0.06 | 0.38 ± 0.05 | 0.01 ± 0.01 | 95.7 | |
| Dexa | ND | 0.02 ± 0.01 | ND | 0.06 ± 0.01 | ND | 98.7 | |
| LPS | 3.2 ± 0.61 | 2.5 ± 0.36 | 1.00 ± 0.12 | 28.79 ± 5.67 | 15.36 ± 3.13 | 99.3 | |
| 1 | 33.1 | 1.48 (53.8) | 1.54 (38.4) | 0.84 (16.0) | 22.52 (21.8) | 11.03 (28.2) | 65.0 |
| 2 | 34.7 | 2.26 (29.4) | 1.61 (35.6) | 1.08 (−8.0) | 26.89 (6.6) | 13.46 (12.4) | 92.2 |
| 3 | 33.1 | 1.46 (54.4) | 1.63 (34.8) | 0.94 (6.0) | 27.47 (4.6) | 11.55 (24.8) | 100.5 |
| 4 | 34.9 | 2.07 (35.3) | 2.12 (15.2) | 1.37 (−37.0) | 31.04 (−7.8) | 19.39 (−26.2) | 103.4 |
| 5 | 34.7 | 2.48 (22.5) | 2.25 (10.0) | 1.02 (−2.0) | 37.96 (−31.9) | 13.81 (10.1) | 103.0 |
| 6 | 33.1 | 2.61 (18.4) | 1.93 (22.8) | 0.82 (18.0) | 17.40 (39.6) | 13.27 (13.6) | 104.5 |
| 7 | 34.7 | 1.99 (37.8) | 0.47 (81.2) | 0.14 (86.0) | 4.79 (83.4) | 16.16 (−5.2) | 101.0 |
| 8 | 34.9 | 2.09 (34.7) | 1.70 (32.0) | 1.05 (−5.0) | 29.01 (−0.8) | 27.93 (−81.8) | 104.3 |
| 9 | 34.9 | 0.66 (79.4) | 0.62 (75.2) | 0.34 (66.0) | 12.65 (56.1) | 7.53 (51.0) | 94.4 |
| 10 | 35.2 | 1.29 (59.8) | 0.94 (62.4) | 0.53 (47.0) | 15.04 (47.8) | 11.85 (22.9) | 102.5 |
| 11 | 30.4 | 1.34 (58.0) | 1.17 (53.2) | 0.56 (44.0) | 23.5 (18.2) | 12.85 (16.3) | 102.5 |
| 12 | 25.5 | 2.26 (29.5) | 1.94 (22.4) | 1.23 (−23.0) | 21.53 (25.2) | 22.15 (−44.2) | 98.9 |
| 13 | 34.7 | 0.47 (85.3) | 0.95 (62.0) | 0.46 (54.0) | 28.81 (−0.1) | 11.83 (23.0) | 97.0 |
| 14 | 33.3 | 0.69 (78.5) | 1.37 (45.2) | 0.49 (51.0) | 22.97 (20.2) | 11.36 (26.0) | 92.5 |
| 15 | 31.8 | 2.79 (12.8) | 1.16 (53.6) | 1.16 (−16.0) | 15.61 (45.8) | 17.60 (−14.6) | 103.6 |
| 16 | 33.3 | 0.64 (80.1) | 0.96 (61.6) | 0.53 (47.0) | 24.40 (15.2) | 15.71 (−2.3) | 106.2 |
| 17 | 33.3 | 0.27 (91.7) | 0.41 (83.6) | 0.39 (61.0) | 15.25 (47.0) | 10.10 (34.2) | 98.0 |
| 18 | 30.3 | 0.48 (85.1) | 0.40 (84.0) | 0.39 (61.0) | 15.29 (46.9) | 7.61 (50.5) | 96.1 |
| 19 | 31.6 | 1.60 (50.0) | 1.50 (40.0) | 0.84 (16.0) | 30.26 (−5.1) | 11.92 (22.4) | 100.7 |
| 20 | 33.1 | 0.75 (76.6) | 0.76 (69.6) | 0.42 (58.0) | 28.52 (0.9) | 9.70 (36.8) | 97.7 |
| 21 | 29.0 | 0.15 (95.3) | 0.40 (84.0) | 0.13 (87.0) | 8.61 (70.1) | 3.64 (76.3) | 101.5 |
| 22 | 30.3 | 1.49 (53.4) | 1.54 (38.4) | 0.84 (16.0) | 32.0 (−11.1) | 11.00 (28.4) | 103.2 |
| 23 | 31.6 | 0.12 (96.2) | 0.61 (75.6) | 0.25 (75.0) | 10.13 (64.8) | 6.41 (58.3) | 98.5 |
| Dexa/LPS | 10.0 | 0.08 ± 0.03 | 0.32 ± 0.03 | 0.25 ± 0.06 | 5.74 ± 3.89 | 5.21 ± 1.67 | 98.5 |
| Compound | Concentration in μM | CD40 MFI (% CD40+ Cells) | CD86 MFI (% CD86+ Cells) | Cell Viability |
|---|---|---|---|---|
| Control | - | 54.7 ± 9.5 (16.4 ± 3.0) | 45.7 ± 5.0 (11.3 ± 1.5) | 99.31 ± 0.7 |
| LPS | - | 267.6 ± 5.5 (62.8± 2.6) | 104.7 ± 2.4 (53.7 ± 3.0) | 100.9 ± 0.5 |
| LPS + 9 | 0.11 | 264.7 ± 13.3 (64.6 ± 0.7) | 98.4 ± 2.8 (55.1 ± 0.2) | 100.9 ± 1.7 |
| 3.49 | 248.0 ± 10.4 (63.7 ± 1.7) | 122.4 ± 7.3 (54.5 ± 1.5) | 102.7 ± 2.4 | |
| 11.03 | 260.9 ± 29.0 (66.6 ± 0.3) | 129.9 ± 8.3 (55.7 ± 1.1) | 98.5 ± 1.7 | |
| 34.9 | 151.1 ± 1.3 a (69.3 ± 6.7) | 106.4 ± 9.9 (44.3 ± 1.4) | 84.9 ± 11.3 | |
| 110.3 | NA | NA | 4.5 ± 2.7 | |
| LPS + 17 | 3.3 | 148.8 ± 49.0 (78.0 ± 3.0) | 100.8 ± 20.0 (53.6 ± 9.3) | 104.6 ± 0.1 |
| 10.5 | 179.5 ± 57.3 (72.7 ± 7.4) | 99.1 ± 23.4 (44.7 ± 10.3) | 102.6 ± 0.4 | |
| 33.3 | 161.9 ± 36.7 (71.3 ± 7.3) | 95.0± 31.1 (45.1 ± 10.2) | 98.0 ± 0.8 | |
| 66.6 | 32.3 ± 21.7 a (32.3 ± 21.7) | 65.5 ± 51.1 (34.0 ± 16.1 c) | 84.0 ± 7.8 | |
| LPS + 18 | 3.03 | 164.9 ± 68.9 a (48.9 ± 11.7) | 96.0 ± 22.8 (42.9 ± 8.1) | 98.4 ± 3.3 |
| 9.6 | 122.8 ± 34.7 a (50.9 ± 10.3) | 65.8 ± 22.9 b (32.0 ± 9.4 c) | 99.0 ± 3.2 | |
| 30.3 | 110.4 ± 30.7 a (45.1 ± 9.9) | 65.2 ± 21.9 b (26.0 ± 7.3 c) | 95.3 ± 2.4 | |
| 60.6 | 17.3 ± 3.8 a (11.0 ± 4.4) | 19.9 ± 7.7 a (6.9 ± 3.2 a) | 81.0 ± 1.6 | |
| 95.6 | NA | NA | 47.5 ± 1.4 | |
| LPS +21 | 2.9 | 216.6 ± 4.1 (39.7 ± 7.6) | 88.2 ± 1.4 (38.3 ± 0.9) | 103.5 ± 0.4 |
| 9.2 | 263.6 ± 0.4 (46.2 ± 8.9) | 72.1 ± 0.5 c (30.2 ± 2.4 c) | 102.4 ± 1.2 | |
| 29.0 | 170.6 ± 0.1 a (38.1 ± 7.5) | 22.9 ± 0.4 a (30.7 ± 1.5 c) | 100.8 ± 1.8 | |
| 91.7 | 0.1 ± 0.1 a (0.05 ±0.05 a) | 0.1 ± 0.1 a (16.7 ± 0.7 a) | 85.5 ± 1.4 | |
| LPS +23 | 3.2 | 157.3 ± 34.0 a (71.0 ± 4.3) | 120.0 ± 3.9 (53.7 ± 2.9) | 100.4 ± 2.7 |
| 10.0 | 161.2 ± 51.5 a (68.7 ± 5.8) | 120.2 ± 3.0 (51.6 ± 3.1) | 99.7 ± 1.9 | |
| 31.6 | 76.5 ± 7.3 a (57.0 ± 7.5) | 82.2 ± 4.4 (38.5 ± 2.6) | 93.2 ± 0.2 | |
| 63.2 | 39.7 ± 3.0 a (30.5 ± 3.5) | 40.4 ± 11.6 a (33.4 ± 1.3 a) | 97.6 ± 6.1 | |
| LPS + Dexa | 10.0 | 149.5 ± 9.2 a (66.0 ± 3.1) | 119.5 ± 5.1 (30.9 ± 2.7 a) | 98.8 ± 0.6 |
| 25.5 | 173.9 ± 10.1 a (61.3 ± 5.5) | 89.4 ± 11.6 (25.9 ± 2.9 a) | 103.3 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra, J.A.; Gilchrist, K.; Tabares-Guevara, J.H.; Betancur-Galvis, L.; Ramirez-Pineda, J.R.; González-Cardenete, M.A. Semisynthetic Abietic and Dehydroabietic Acid Derivatives and Triptoquinone Epimers Interfere with LPS-Triggered Activation of Dendritic Cells. Molecules 2022, 27, 6684. https://doi.org/10.3390/molecules27196684
Sierra JA, Gilchrist K, Tabares-Guevara JH, Betancur-Galvis L, Ramirez-Pineda JR, González-Cardenete MA. Semisynthetic Abietic and Dehydroabietic Acid Derivatives and Triptoquinone Epimers Interfere with LPS-Triggered Activation of Dendritic Cells. Molecules. 2022; 27(19):6684. https://doi.org/10.3390/molecules27196684
Chicago/Turabian StyleSierra, Jelver A., Katherine Gilchrist, Jorge H. Tabares-Guevara, Liliana Betancur-Galvis, Jose R. Ramirez-Pineda, and Miguel A. González-Cardenete. 2022. "Semisynthetic Abietic and Dehydroabietic Acid Derivatives and Triptoquinone Epimers Interfere with LPS-Triggered Activation of Dendritic Cells" Molecules 27, no. 19: 6684. https://doi.org/10.3390/molecules27196684