Efficient Oxidative Dearomatisations of Substituted Phenols Using Hypervalent Iodine (III) Reagents and Antiprotozoal Evaluation of the Resulting Cyclohexadienones against T. b. rhodesiense and P. falciparum Strain NF54
Abstract
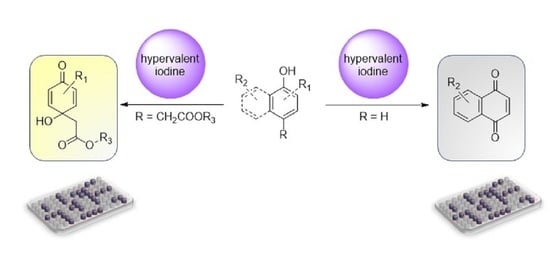
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiprotozoal Activity
2.3. Physicochemical Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Dearomatization with µ-oxo dimer 1. Method A:
3.1.3. General Procedure for the Dearomatisation with PIDA. Method B:
3.2. Biological Testing
3.2.1. Assay for In Vitro Antimalarial Activity
3.2.2. Assay for In Vitro Trypanocidal Activity
3.2.3. Assay for Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Milackova, I.; Prnova, M.S.; Majekova, M.; Sotnikova, R.; Stasko, M.; Kovacikova, L.; Banerjee, S.; Veverka, M.; Stefek, M. 2-Chloro-1,4-naphthoquinone derivative of quercetin as an inhibitor of aldose reductase and anti-inflammatory agent. J. Enzyme Inhib. Med. Chem. 2015, 30, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sychrova, A.; Kolarikova, I.; Zemlicka, M.; Smejkal, K. Natural compounds with dual antimicrobial and anti-inflammatory effects. Phytochem. Rev. 2020, 19, 1471–1502. [Google Scholar] [CrossRef]
- Siddamurthi, S.; Gutti, G.; Jana, S.; Kumar, A.; Singh, S.K. Anthraquinone: A promising scaffold for the discovery and development of therapeutic agents in cancer therapy. Future Med. Chem. 2020, 12, 1037–1069. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Junior, E.N.; Jardim, G.A.M.; Jacob, C.; Dhawa, U.; Ackermann, L.; de Castro, S.L. Synthesis of quinones with highlighted biological applications: A critical update on the strategies towards bioactive compounds with emphasis on lapachones. Eur. J. Med. Chem. 2019, 179, 863–915. [Google Scholar] [CrossRef] [PubMed]
- Roa-Linares, V.C.; Miranda-Brand, Y.; Tangarife-Castano, V.; Ochoa, R.; Garcia, P.A.; Castro, M.A.; Betancur-Galvis, L.; Feliciano, A.S. Anti-herpetic, anti-dengue and antineoplastic activities of simple and heterocycle-fused derivatives of terpenyl-1,4-naphthoquinone and 1,4-anthraquinone. Molecules 2019, 24, 1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, D.; Ray, R.; Hazra, B. Antitubercular and Antibacterial Activity of Quinonoid Natural Products Against Multi-Drug Resistant Clinical Isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef]
- Halicki, P.C.B.; Ferreira, L.A.; de Moura, K.C.G.; Carneiro, P.F.; Del Rio, K.P.; Carvalho, T.D.S.C.; Pinto, M.d.C.F.R.; da Silva, P.E.A.; Ramos, D.F. Naphthoquinone derivatives as scaffold to develop new drugs for tuberculosis treatment. Front. Microbiol. 2018, 9, 673. [Google Scholar] [CrossRef] [Green Version]
- Futuro, D.O.; Ferreira, V.F.; Ferreira, P.G.; Nicoletti, C.D.; Borba-Santos, L.P.; Rozental, S.; Silva, F.C.D. The Antifungal Activity of Naphthoquinones: An Integrative Review. An. Acad. Bras. Cienc. 2018, 90, 1187–1214. [Google Scholar] [CrossRef] [Green Version]
- Ravichandiran, P.; Maslyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Park, B.-H.; Han, M.-K.; Yoo, D.J. Synthesis and Antimicrobial Evaluation of 1,4-Naphthoquinone Derivatives as Potential Antibacterial Agents. ChemistryOpen 2019, 8, 589–600. [Google Scholar] [CrossRef]
- Prati, F.; Bergamini, C.; Molina, M.T.; Falchi, F.; Cavalli, A.; Kaiser, M.; Brun, R.; Fato, R.; Bolognesi, M.L. 2-Phenoxy-1,4-naphthoquinones: From a Multitarget Antitrypanosomal to a Potential Antitumor Profile. J. Med. Chem. 2015, 58, 6422–6434. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Beteck, R.M.; Legoabe, L.J. Antimalarial application of quinones: A recent update. Eur. J. Med. Chem. 2021, 210, 113084. [Google Scholar] [CrossRef] [PubMed]
- Baillie, T.A. Targeted Covalent Inhibitors for Drug Design. Angew. Chem. Int. Ed. 2016, 55, 13408–13421. [Google Scholar] [CrossRef] [PubMed]
- Oramas-Royo, S.; Lopez-Rojas, P.; Amesty, A.; Gutierrez, D.; Flores, N.; Martin-Rodriguez, P.; Fernandez-Perez, L.; Estevez-Braun, A. Synthesis and antiplasmodial activity of 1,2,3-triazole-naphthoquinone conjugates. Molecules 2019, 24, 3917. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.; Berry, J.M.; Bradshaw, T.D.; Burger, A.M.; Seaton, A.; Wang, B.; Westwell, A.D.; Stevens, M.F.G. 4-Substituted 4-Hydroxycyclohexa-2,5-dien-1-ones with Selective Activities against Colon and Renal Cancer Cell Lines. J. Med. Chem. 2003, 46, 532–541. [Google Scholar] [CrossRef]
- Hughes, L.M.; Lanteri, C.A.; O’Neil, M.T.; Johnson, J.D.; Gribble, G.W.; Trumpower, B.L. Design of antiparasitic and antifungal hydroxy-naphthoquinones that are less susceptible to drug resistance. Mol. Biochem. Parasitol. 2011, 177, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Barradas, S.; Hernandez-Torres, G.; Urbano, A.; Carreno, M.C. Total Synthesis of Natural p-Quinol Cochinchinenone. Org. Lett. 2012, 14, 5952–5955. [Google Scholar] [CrossRef]
- Morais, T.R.; Romoff, P.; Favero, O.A.; Reimao, J.Q.; Lourenco, W.C.; Tempone, A.G.; Hristov, A.D.; Di, S.S.M.; Lago, J.H.G.; Sartorelli, P.; et al. Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacalia desiderabilis (Vell.) Cuatrec. (Asteraceae). Parasitol. Res. 2012, 110, 95–101. [Google Scholar] [CrossRef]
- Kalt, M.-M.; Schuehly, W.; Ochensberger, S.; Solnier, J.; Bucar, F.; Saf, R.; Kaiser, M.; Presser, A. Palladium-catalysed synthesis of arylnaphthoquinones as antiprotozoal and antimycobacterial agents. Eur. J. Med. Chem. 2020, 207, 112837. [Google Scholar] [CrossRef]
- Quideau, S.; Pouysegu, L.; Deffieux, D. Oxidative dearomatization of phenols. Why, how and what for? Synlett 2008, 4, 467–495. [Google Scholar] [CrossRef]
- Santhosh Reddy, R.; Shaikh, T.M.; Rawat, V.; Karabal, P.U.; Dewkar, G.; Suryavanshi, G.; Sudalai, A. A novel synthesis and characterization of titanium superoxide and its application in organic oxidative processes. Catal. Surv. Asia 2010, 14, 21–32. [Google Scholar] [CrossRef]
- Roche, S.P.; Porco, J.A., Jr. Dearomatization Strategies in the Synthesis of Complex Natural Products. Angew. Chem. Int. Ed. 2011, 50, 4068–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Li, B.; Feng, Z.; Kim, Y.A.; Endo, M.; Su, D.S. Efficient Metal-Free Catalytic Reaction Pathway for Selective Oxidation of Substituted Phenols. ACS Catal. 2015, 5, 5921–5926. [Google Scholar] [CrossRef]
- Shanmugam, V.M.; Kulangiappar, K.; Ramaprakash, M.; Vasudevan, D.; Senthil Kumar, R.; Velayutham, D.; Raju, T. Electrochemical synthesis of quinones and other derivatives in biphasic medium. Tetrahedron Lett. 2017, 58, 2294–2297. [Google Scholar] [CrossRef]
- Dohi, T.; Nakae, T.; Takenaga, N.; Uchiyama, T.; Fukushima, K.; Fujioka, H.; Kita, Y. μ-oxo-bridged hypervalent iodine(III) compound as an extreme oxidant for aqueous oxidations. Synthesis 2012, 44, 1183–1189. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef]
- Chandra, G.; Patel, S. Molecular Complexity from Aromatics: Recent Advances in the Chemistry of para-Quinol and Masked para-Quinone Monoketal. ChemistrySelect 2020, 5, 12885–12909. [Google Scholar] [CrossRef]
- Harned, A.M. Asymmetric oxidative dearomatizations promoted by hypervalent iodine(III) reagents: An opportunity for rational catalyst design? Tetrahedron Lett. 2014, 55, 4681–4689. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Li, G.; Hong, L.; Wang, R. Asymmetric dearomatization of phenols. Org. Biomol. Chem. 2016, 14, 2164–2176. [Google Scholar] [CrossRef]
- Wu, W.-T.; Zhang, L.; You, S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 2016, 45, 1570–1580. [Google Scholar] [CrossRef]
- Baker Dockrey, S.A.; Lukowski, A.L.; Becker, M.R.; Narayan, A.R.H. Biocatalytic site- and enantioselective oxidative dearomatization of phenols. Nat. Chem. 2018, 10, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhdankin, V.V.; Stang, P.J. Chemistry of Polyvalent Iodine. Chem. Rev. 2008, 108, 5299–5358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouysegu, L.; Deffieux, D.; Quideau, S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron 2010, 66, 2235–2261. [Google Scholar] [CrossRef]
- Kraszewski, K.; Tomczyk, I.; Drabinska, A.; Bienkowski, K.; Solarska, R.; Kalek, M. Mechanism of Iodine(III)-Promoted Oxidative Dearomatizing Hydroxylation of Phenols: Evidence for a Radical-Chain Pathway. Chem. Eur. J. 2020, 26, 11584–11592. [Google Scholar] [CrossRef] [PubMed]
- Felpin, F.-X. Oxidation of 4-arylphenol trimethylsilyl ethers to p-arylquinols using hypervalent iodine(III) reagents. Tetrahedron Lett. 2007, 48, 409–412. [Google Scholar] [CrossRef]
- You, Z.; Hoveyda, A.H.; Snapper, M.L. Catalytic enantioselective silylation of acyclic and cyclic triols: Application to total syntheses of cleroindicins D, F, and C. Angew. Chem. Int. Ed. 2009, 48, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Tello-Aburto, R.; Kalstabakken, K.A.; Volp, K.A.; Harned, A.M. Regioselective and stereoselective cyclizations of cyclohexadienones tethered to active methylene groups. Org. Biomol. Chem. 2011, 9, 7849–7859. [Google Scholar] [CrossRef]
- McKillop, A.; McLaren, L.; Taylor, R.J.K. A simple and efficient procedure for the preparation of p-quinols by hypervalent iodine oxidation of phenols and phenol tripropylsilyl ethers. J. Chem. Soc., Perkin Trans. 1994, 15, 2047–2048. [Google Scholar] [CrossRef]
- Jo, H.; Choi, M.; Viji, M.; Lee, Y.H.; Kwak, Y.-S.; Lee, K.; Choi, N.S.; Lee, Y.-J.; Lee, H.; Hong, J.T.; et al. Concise Synthesis of Broussonone A. Molecules 2015, 20, 15966–15975. [Google Scholar] [CrossRef] [Green Version]
- Dohi, T.; Uchiyama, T.; Yamashita, D.; Washimi, N.; Kita, Y. Efficient phenolic oxidations using μ-oxo-bridged phenyliodine trifluoroacetate. Tetrahedron Lett. 2011, 52, 2212–2215. [Google Scholar] [CrossRef]
- Lion, C.; Matthews, C.S.; Wells, G.; Bradshaw, T.D.; Stevens, M.F.G.; Westwell, A.D. Antitumor properties of fluorinated benzothiazole-substituted hydroxycyclohexa-2,5-dienones (‘quinols’). Bioorg. Med. Chem. Lett. 2006, 16, 5005–5008. [Google Scholar] [CrossRef] [PubMed]
- Barret, R.; Daudon, M. Oxidation of phenols to quinones by bis(trifluoroacetoxy)iodobenzene. Tetrahedron Lett. 1990, 31, 4871–4872. [Google Scholar] [CrossRef]
- Shahinas, D.; Liang, M.; Datti, A.; Pillai, D.R. A Repurposing Strategy Identifies Novel Synergistic Inhibitors of Plasmodium falciparum Heat Shock Protein 90. J. Med. Chem. 2010, 53, 3552–3557. [Google Scholar] [CrossRef] [PubMed]
- Blumenstiel, K.; Schoneck, R.; Yardley, V.; Croft, S.L.; Krauth-Siegel, R.L. Nitrofuran drugs as common subversive substrates of Trypanosoma cruzi lipoamide dehydrogenase and trypanothione reductase. Biochem. Pharmacol. 1999, 58, 1791–1799. [Google Scholar] [CrossRef]
- Salmon-Chemin, L.; Buisine, E.; Yardley, V.; Kohler, S.; Debreu, M.-A.; Landry, V.; Sergheraert, C.; Croft, S.L.; Krauth-Siegel, R.L.; Davioud-Charvet, E. 2- and 3-Substituted 1,4-Naphthoquinone Derivatives as Subversive Substrates of Trypanothione Reductase and Lipoamide Dehydrogenase from Trypanosoma cruzi: Synthesis and Correlation between Redox Cycling Activities and in Vitro Cytotoxicity. J. Med. Chem. 2001, 44, 548–565. [Google Scholar] [CrossRef]
- Nwaka, S.; Ramirez, B.; Brun, R.; Maes, L.; Douglas, F.; Ridley, R. Advancing drug innovation for neglected diseases-criteria for lead progression. PLoS Negl. Trop. Dis. 2009, 3, e440. [Google Scholar] [CrossRef]
- Katsuno, K.; Burrows, J.n.; Duncan, K.; van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef]
- Samby, K.; Willis, P.A.; Burrows, J.N.; Laleu, B.; Webborn, P.J.H. Actives from MMV Open Access Boxes? A suggested way forward. PLoS Pathog. 2021, 17, e1009384. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257. [Google Scholar] [CrossRef] [Green Version]
- Sena Pereira, V.S. de; Silva de Oliveira, Claudio Bruno; Fumagalli, F.; Da Silva Emery, F.; Da Silva, N.B.; Andrade-Neto, V.F. de. Cytotoxicity, hemolysis and in vivo acute toxicity of 2-hydroxy-3-anilino-1,4-naphthoquinone derivatives. Toxicol. Rep. 2016, 3, 756–762. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Jalal, R.; Singh, P.P.; Majoral, J.-P.; Vishwakarma, R.A. Present drug-likeness filters in medicinal chemistry during the hit and lead optimization process: How far can they be simplified? Drug Discov. Today 2018, 23, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Agoni, C.; Olotu, F.A.; Ramharack, P.; Soliman, M.E. Druggability and drug-likeness concepts in drug design: Are biomodelling and predictive tools having their say? J. Mol. Model. 2020, 26, 120. [Google Scholar] [CrossRef]
- Leeson, P.D.; Bento, A.P.; Gaulton, A.; Hersey, A.; Manners, E.J.; Radoux, C.J.; Leach, A.R. Target-Based Evaluation of “Drug-Like” Properties and Ligand Efficiencies. J. Med. Chem. 2021, 64, 7210–7230. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Gallego, R.A.; Edwards, M.P. Lipophilic efficiency as an important metric in drug design. J. Med. Chem. 2018, 61, 6401–6420. [Google Scholar] [CrossRef]
- Scott, J.S.; Waring, M.J. Practical application of ligand efficiency metrics in lead optimisation. Bioorg. Med. Chem. 2018, 26, 3006–3015. [Google Scholar] [CrossRef] [Green Version]
- Tinworth, C.P.; Young, R.J. Facts, Patterns, and Principles in Drug Discovery: Appraising the Rule of 5 with Measured Physicochemical Data. J. Med. Chem. 2020, 63, 10091–10108. [Google Scholar] [CrossRef]
- Charman, S.A.; Andreu, A.; Barker, H.; Blundell, S.; Campbell, A.; Campbell, M.; Chen, G.; Chiu, F.C.K.; Crighton, E.; Katneni, K.; et al. An in vitro toolbox to accelerate anti-malarial drug discovery and development. Malar. J. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad-Zapatero, C. Ligand efficiency indices for effective drug discovery: A unifying vector formulation. Expert Opin. Drug Discov. 2021, 16, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Keserü, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef]
- Gallos, J.; Varvoglis, A.; Alcock, N.W. Oxo-bridged compounds of iodine(III): Syntheses, structure, and properties of μ-oxobis[trifluoroacetato(phenyl)iodine]. J. Chem. Soc. Perkin Trans. 1985, 757–763. [Google Scholar] [CrossRef]
- Hirukawa, M.; Zhang, M.; Echenique-Diaz, L.M.; Mizota, K.; Ohdachi, S.D.; Begue-Quiala, G.; Delgado-Labanino, J.L.; Gamez-Diez, J.; Alvarez-Lemus, J.; Machado, L.G.; et al. Isolation and structure-activity relationship studies of jacaranones: Anti-inflammatory quinoids from the Cuban endemic plant Jacaranda arborea (Bignoniaceae). Tetrahedron Lett. 2020, 61, 152005. [Google Scholar] [CrossRef]
- Shestak, O.P.; Novikov, V.L.; Ivanova, E.P.; Gorshkova, N.M. Synthesis and antimicrobial activity of [3,5-dibromo(dichloro)-1-hydroxy-4-oxocyclohexa-2,5-dien-1-yl]acetic acids and their derivatives. Pharm. Chem. J. 2001, 35, 366–369. [Google Scholar] [CrossRef]
- Zhou, Z.-S.; He, X.-H. Convenient catalyzed spirocyclization of 4-(2-carboxyethyl)phenols. J. Chem. Res. 2017, 41, 57–59. [Google Scholar] [CrossRef]
- Fasi, L.; Di Meo, F.; Kuo, C.-Y.; Stojkovic Buric, S.; Martins, A.; Kusz, N.; Beni, Z.; Dekany, M.; Balogh, G.T.; Pesic, M.; et al. Antioxidant-Inspired Drug Discovery: Antitumor Metabolite Is Formed in Situ from a Hydroxycinnamic Acid Derivative upon Free-Radical Scavenging. J. Med. Chem. 2019, 62, 1657–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murahashi, S.; Miyaguchi, N.; Noda, S.; Naota, T.; Fujii, A.; Inubushi, Y.; Komiya, N. Ruthenium-Catalyzed Oxidative Dearomatization of Phenols to 4-(tert-Butylperoxy)-cyclohexadienones: Synthesis of 2-Substituted Quinones from p-Substituted Phenols. Eur. J. Org. Chem. 2011, 2011, 5355–5365. [Google Scholar] [CrossRef]
- Periasamy, M.; Bhatt, V.M. A new 1,2-shift in the oxidation of aromatic rings. Tetrahedron Lett. 1977, 18, 2357–2360. [Google Scholar] [CrossRef]
- Wellauer, J.; Miladinov, D.; Buchholz, T.; Schuetz, J.; Stemmler, R.T.; Medlock, J.A.; Bonrath, W.; Sparr, C. Organophotocatalytic Aerobic Oxygenation of Phenols in a Visible-Light Continuous-Flow Photoreactor. Chem. Eur. J. 2021, 27, 9748–9752. [Google Scholar] [CrossRef] [PubMed]
- Bunge, A.; Hamann, H.-J.; McCalmont, E.; Liebscher, J. Enantioselective epoxidation of 2-substituted 1,4-naphthoquinones using gem-dihydroperoxides. Tetrahedron Lett. 2009, 50, 4629–4632. [Google Scholar] [CrossRef]
- Ren, J.; Lu, L.; Xu, J.; Yu, T.; Zeng, B.-B. Selective Oxidation of 1-Tetralones to 1,2-Naphthoquinones with IBX and to 1,4-Naphthoquinones with Oxone and 2-Iodobenzoic Acid. Synthesis 2015, 47, 2270–2280. [Google Scholar] [CrossRef]
- Shu, C.; Shi, C.-Y.; Sun, Q.; Zhou, B.; Li, T.-Y.; He, Q.; Lu, X.; Liu, R.-S.; Ye, L.-W. Generation of Endocyclic Vinyl Carbene Complexes via Gold-Catalyzed Oxidative Cyclization of Terminal Diynes: Toward Naphthoquinones and Carbazolequinones. ACS Catal. 2019, 9, 1019–1025. [Google Scholar] [CrossRef]
- Matile, H.; Richard, J.; Pink, L. Plasmodium falciparum malaria parasite cultures and their use in immunology. In Immunological Methods, Volume IV; Lefkovits, I., Pernis, B., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 221–234. ISBN 1483242536. [Google Scholar]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Presser, A.; Lainer, G.; Kretschmer, N.; Schuehly, W.; Saf, R.; Kaiser, M.; Kalt, M.-M. Synthesis of jacaranone-derived nitrogenous cyclohexadienones and their antiproliferative and antiprotozoal activities. Molecules 2018, 23, 2902. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Method a | Time b | Yield/% c |
| 1 |  2a |  4a | A B C D | 10 20 30 30 | 45 42 39 34 |
| 2 |  2b |  4b | A B | 10 20 | 32 29 |
| 3 |  2c |  4c | A B | 10 20 | 63 53 |
| 4 |  2d |  4d | A B | 10 20 | 62 41 |
| 5 |  2e |  4e | A B | 10 20 | 68 47 |
| 6 |  2f |  4f | A B | 10 20 | 62 34 |
| 7 |  2g |  4g | A B | 20 20 | 67 60 |
| 8 |  2h |  4h | A B | 20 20 | 8 6 |
| 9 |  2i |  4i | A B | 10 20 | 15 11 |
| 10 |  2j |  4j | A B | 10 20 | 0 0 |
| 11 |  2k |  4k | A B | 10 20 | 0 0 |
| 12 |  2l |  4l | A B | 10 20 | 0 0 |
| 13 |  3a |  5a | A B C D | 90 90 60 90 | 75 76 28 46 |
| 14 |  3b |  5b | A B | 90 90 | 73 71 |
| 15 |  3c |  5c | A B | 90 90 | 65 82 |
| 16 |  3d |  5d | A B | 90 90 | 74 87 |
| 17 |  3e |  5e | A B | 90 150 | 71 82 |
| ID No. | P. falc.a | SI b | T. b. rhod. c | SI b | Cyt. L6 d | Chemical | log P | log D7.4 |
|---|---|---|---|---|---|---|---|---|
| IC50 μM | IC50 μM | IC50 μM | Structure | |||||
| Chl. | 0.002 | 45,500 | 91.1 | |||||
| Mel. | 0.004 | 6050 | 24.2 | |||||
| Pod. | 0.007 | |||||||
| 4a | 0.969 | 0.40 | 0.588 | 0.66 | 0.391 |  | 0.11 | 0.19 |
| 4b | 0.644 | 1.02 | 0.078 | 8.42 | 0.657 |  | −0.37 | 0.12 |
| 4c | 0.837 | 0.53 | 0.047 | 9.36 | 0.440 |  | 0.01 | 0.58 |
| 4d | 0.301 | 0.71 | 0.042 | 5.09 | 0.214 |  | 0.28 | 0.75 |
| 4e | 0.245 | 0.47 | 0.01 | 11.6 | 0.116 |  | −0.09 | 0.97 |
| 4f | 0.391 | 0.51 | 0.024 | 8.29 | 0.199 |  | 0.46 | 1.30 |
| 4g | 8.16 | >12.3 | 4.87 | >20.5 | >100 |  | 0.36 | 0.48 |
| 4h | 2.75 | 0.15 | 4.89 | 0.09 | 0.425 |  | 0.93 | 1.00 |
| 4i | 0.657 | 0.87 | 0.108 | 5.27 | 0.569 |  | 1.28 | 0.87 |
| 5c | 0.917 | 23.97 | 0.08 | 274.75 | 21.98 |  | 2.93 | 3.47 |
| 5d | 0.677 | 3.21 | 0.093 | 23.33 | 2.17 |  | 1.18 | 1.64 |
| 5e | 0.689 | 6.97 | 0.166 | 28.92 | 4.80 |  | 1.32 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiber, N.; Blaser, G.; Pferschy-Wenzig, E.-M.; Kaiser, M.; Mäser, P.; Presser, A. Efficient Oxidative Dearomatisations of Substituted Phenols Using Hypervalent Iodine (III) Reagents and Antiprotozoal Evaluation of the Resulting Cyclohexadienones against T. b. rhodesiense and P. falciparum Strain NF54. Molecules 2022, 27, 6559. https://doi.org/10.3390/molecules27196559
Scheiber N, Blaser G, Pferschy-Wenzig E-M, Kaiser M, Mäser P, Presser A. Efficient Oxidative Dearomatisations of Substituted Phenols Using Hypervalent Iodine (III) Reagents and Antiprotozoal Evaluation of the Resulting Cyclohexadienones against T. b. rhodesiense and P. falciparum Strain NF54. Molecules. 2022; 27(19):6559. https://doi.org/10.3390/molecules27196559
Chicago/Turabian StyleScheiber, Nina, Gregor Blaser, Eva-Maria Pferschy-Wenzig, Marcel Kaiser, Pascal Mäser, and Armin Presser. 2022. "Efficient Oxidative Dearomatisations of Substituted Phenols Using Hypervalent Iodine (III) Reagents and Antiprotozoal Evaluation of the Resulting Cyclohexadienones against T. b. rhodesiense and P. falciparum Strain NF54" Molecules 27, no. 19: 6559. https://doi.org/10.3390/molecules27196559