Synthesis and Antifungal Activity of New butenolide Containing Methoxyacrylate Scaffold
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. The X-ray Structure Analysis of IV-6
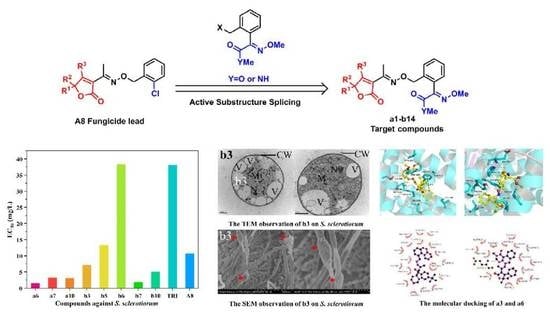
2.3. The Antifungal Activities
2.4. The Docking Study
3. Materials and Methods
3.1. General Information
3.2. The Synthesis of Intermediates III-1~III-14 and IV-1~IV-14
3.3. The Synthesis of Target Compounds V-1~VI-14
3.4. Determination of the In Vitro Antifungal Activity of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ouyang, Y.; Huang, J.; Wang, Y.; Zhong, H.; Song, B.; Hao, G. In Silico resources of drug-likeness as a mirror: What are we lacking in pesticide-likeness? J. Agric. Food Chem. 2021, 69, 10761–10773. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Li, Y.; Zhang, S.; Shi, P.; Li-Byarlay, H.; Luo, S. Pesticide residues in beebread and honey in Apis cerana and their hazards to honey bees and human. Ecotoxicol. Environ. Saf. 2022, 238, 113574. [Google Scholar] [CrossRef] [PubMed]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Manea-Karga, E.; Machera, K. Occurrence and human health risk assessment of mineral elements and pesticides residues in bee pollen. Food Chem. Toxicol. 2022, 161, 112826. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Chen, L.; Zhang, Y.; Gao, W.; Chen, L.; Wang, D.; Tang, L.; Wang, Z.; Wang, N.N.; Fan, Z. Methoxyacrylate fungicide candidate CL-15C also functions as a plant elicitor in Arabidopsis thaliana and Oryza sativa L. J. Agric. Food Chem. 2022, 70, 3142–3150. [Google Scholar] [CrossRef]
- Clough, J.M.; Evans, D.A.; de Fraine, P.J.; Fraser, T.E.M.; Godfrey, C.R.A.; Youle, D. Role of natural products in pesticide discovery. In natural and engineered pest management agents. J. Am. Chem. Soc. 1993, 551, 37–53. [Google Scholar]
- Kornsakulkarn, J.; Palasarn, S.; Choowong, W.; Thongpanchang, T.; Boonyuen, N.; Choeyklin, R.; Boonpratuang, T.; Isaka, M.; Thongpanchang, C. Antimalarial 9-methoxystrobilurins, oudemansins, and related polyketides from cultures of Basidiomycete Favolaschia Species. J. Nat. Prod. 2020, 83, 905–917. [Google Scholar] [CrossRef]
- Panter, F.; Krug, D.; Müller, R. Novel methoxymethacrylate natural products uncovered by statistics-based mining of the Myxococcus fulvus secondary metabolome. ACS Chem. Biol. 2019, 14, 88–98. [Google Scholar] [CrossRef]
- Shi, G.; Zhao, Z.; Zhang, X. Ethyl 3-fluoro-3-(tributylstannyl)-2-methoxyacrylate: Preparation and palladium/copper-cocatalyzed cross-coupling reactions as a novel route to β-fluoro-α-keto acid derivatives. J. Org. Chem. 1995, 60, 6608–6611. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, F.; Zhang, M.; Liu, Z.; Huang, W.; Yang, G. Synthesis, fungicidal, and insecticidal activities of β-methoxyacrylate-containing N-acetyl pyrazoline derivatives. J. Agric. Food Chem. 2008, 56, 10767–10773. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest. Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Beautement, K.; Clough, J.M.; de Fraine, P.J.; Godfrey, C.R.A. Fungicidal β-methoxyacrylates. In Synthesis and Chemistry of Agrochemicals IV. J. Am. Chem. Soc. 1995, 584, 326–342. [Google Scholar]
- Liu, A.; Wang, X.; Chen, C.; Pei, H.; Mao, C.; Wang, Y.; He, H.; Huang, L.; Liu, X.; Hu, Z.; et al. The discovery of HNPC-A3066: A novel strobilurin acaricide. Pest. Manag. Sci. 2009, 65, 229–234. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Dixon, E.W.; Vincelli, P.; Farman, M.L. Field resistance to strobilurin (QoI) fungicides in Pyricularia grisea caused by mutations in the mitochondrial cytochrome b gene. Phytopathology 2003, 93, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.S.; Auclair, C.; Gredt, M.; Leroux, P. First occurrence of resistance to strobilurin fungicides in Microdochium nivale and Microdochium majus from French naturally infected wheat grains. Pest. Manag. Sci. 2009, 65, 906–915. [Google Scholar] [CrossRef]
- Lorsbach, B.A.; Sparks, T.C.; Cicchillo, R.M.; Garizi, N.V.; Hahn, D.R.; Meyer, K.G. Natural products: A strategic lead generation approach in crop protection discovery. Pest. Manag. Sci. 2019, 75, 2301–2309. [Google Scholar] [CrossRef]
- Manam, R.R.; Macherla, V.R.; Tsueng, G.; Dring, C.W.; Weiss, J.; Neuteboom, S.T.C.; Lam, K.S.; Potts, B.C. Antiprotealide is a natural product. J. Nat. Prod. 2009, 72, 295–297. [Google Scholar] [CrossRef]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Impact of natural products on discovery of, and innovation in, crop protection compounds. Pest. Manag. Sci. 2022, 78, 399–408. [Google Scholar] [CrossRef]
- Wang, P.; Liu, A.L.; An, Z.; Li, Z.H.; Du, G.H.; Qin, H.L. Novel alkaloids from the roots of Stemona sessilifolia. Chem. Biodivers. 2007, 4, 523–530. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Development of potential antitumor agents from the scaffolds of plant-derived terpenoid lactones. J. Med. Chem. 2020, 63, 15410–15448. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Nanda, L.N.; Shukla, M.K. Donor–acceptor substituted cyclopropane to butanolide and butenolide natural products: Enantiospecific first total synthesis of (+)-hydroxyancepsenolide. Org. Lett. 2014, 16, 6424–6427. [Google Scholar] [CrossRef]
- Dorta, E.; Cueto, M.; Díaz-Marrero, A.R.; Darias, J. Stypolactone, an interesting diterpenoid from the brown alga Stypopodium zonale. Tetrahedron Lett. 2002, 43, 9043–9046. [Google Scholar] [CrossRef]
- Murakami, T.; Morikawa, Y.; Hashimoto, M.; Okuno, T.; Harada, Y. Lambertellols A and B, novel 3,4-dihydronaphthalen-1(2H)-ones with spiro-butenolide produced by Lambertella. Org. Lett. 2004, 6, 157–160. [Google Scholar] [CrossRef]
- Liu, M.; Lin, S.; Gan, M.; Chen, M.; Li, L.; Wang, S.; Zi, J.; Fan, X.; Liu, Y.; Si, Y.; et al. Yaoshanenolides A and B: New spirolactones from the bark of Machilus yaoshansis. Org. Lett. 2012, 14, 1004–1007. [Google Scholar] [CrossRef]
- Kotzabasaki, V.; Vassilikogiannakis, G.; Stratakis, M. Total synthesis and structural revision of (+)-yaoshanenolide B. Org. Lett. 2016, 18, 4982–4985. [Google Scholar] [CrossRef]
- Tian, P.; Liu, D.; Liu, Z.; Shi, J.; He, W.; Qi, P.; Chen, J.; Song, B. Design, synthesis, and insecticidal activity evaluation of novel 4-(N,N-diarylmethylamines)furan-2(5H)-one derivatives as potential acetylcholine receptor insecticides. Pest. Manag. Sci. 2019, 75, 427–437. [Google Scholar] [CrossRef]
- Han, Q.; Wu, N.; Li, H.-L.; Zhang, J.-Y.; Li, X.; Deng, M.-F.; Zhu, K.; Wang, J.-E.; Duan, H.-X.; Yang, Q. A piperine-based scaffold as a novel starting point to develop inhibitors against the potent molecular target OfChtI. J. Agric. Food Chem. 2021, 69, 7534–7544. [Google Scholar] [CrossRef]
- Mo, X.; Li, Q.; Ju, J. Naturally ocurring tetramic acid products: Isolation, structure elucidation and biological activity. RSC Adv. 2014, 4, 50566–50593. [Google Scholar] [CrossRef]
- Elagamy, A.; Shaw, R.; Panwar, R.; Shally; Ram, V.J.; Pratap, R. Synthesis of highly functionalized spirobutenolides via a nitroalkane-mediated ring contraction of 2-oxobenzo[h]chromenes through denitration. J. Org. Chem. 2019, 84, 1154–1161. [Google Scholar] [CrossRef]
- Liu, Y.X.; Cui, Z.P.; Zhao, H.P.; Li, Y.H.; Gu, Y.C.; Wang, Q.M. Synthesis and biological activities of 3-substituted analogues of tenuazonic acid. J. Heterocycl. Chem. 2014, 51, 209–215. [Google Scholar] [CrossRef]
- Tu, S.; Xu, L.H.; Ye, L.Y.; Wang, X.; Sha, Y.; Xiao, Z.Y. Synthesis and fungicidal activities of novel indene-substituted oxime ether strobilurins. J. Agric. Food Chem. 2008, 56, 5247–5253. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Li, X.-C.; Jacob, M.R.; Zhang, Q.; Khan, S.I.; Ferreira, D.; Clark, A.M. Synthesis, antifungal activity, and structure−activity relationships of coruscanone A analogues. J. Med. Chem. 2006, 49, 7877–7886. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.X. Toxicity and efficacy of spiromesifen, a tetronic acid insecticide, against sweetpotato whitefly (homoptera: Aleyrodidae) on melons and collards. Crop Prot. 2004, 23, 505–513. [Google Scholar] [CrossRef]
- Nauen, R.; Bretschneider, T.; Elbert, A.; Fischer, R.; Tieman, R. Spirodiclofen and Spiromesifen. Pestic. Outlook 2003, 14, 243–246. [Google Scholar] [CrossRef]
- Chen, X.; Meng, Z.; Song, Y.; Zhang, Q.; Ren, L.; Guan, L.; Ren, Y.; Fan, T.; Shen, D.; Yang, Y. Adsorption and desorption behaviors of spirotetramat in various soils and its interaction mechanism. J. Agric. Food Chem. 2018, 66, 12471–12478. [Google Scholar] [CrossRef]
- Muehlebach, M.; Buchholz, A.; Zambach, W.; Schaetzer, J.; Daniels, M.; Hueter, O.; Kloer, D.P.; Lind, R.; Maienfisch, P.; Pierce, A.; et al. Spiro N-methoxy piperidine ring containing aryldiones for the control of sucking insects and mites: Discovery of spiropidion. Pest. Manag. Sci. 2020, 76, 3440–3450. [Google Scholar] [CrossRef]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest. Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef] [Green Version]
- Niehs, S.P.; Kumpfmueller, J.; Dose, B. Insect-Associated bacteria assemble the antifungal butenolide gladiofungin by non-canonical polyketide chain termination. Angew. Chem. Int. Ed. 2020, 59, 23122–23126. [Google Scholar] [CrossRef]
- Novak, P.; Pour, M.; Spulak, M.; Votruba, I.; Kotora, M. Extension of the library of biologically active γ-alkylidene butenolides. Synthesis 2008, 2008, 3465–3472. [Google Scholar]
- Tang, B.; Guan, A.; Zhao, Y.; Jiang, J.; Wang, M.; Zhou, L. Synthesis and fungicidal activity of (E)-5-[1-(2-oxo-1-oxaspiro[4,5]dec/non-3-en-3-yl)ethylidene]-2-aminoimidazolin-4-one derivatives. Chin. J. Chem. 2017, 35, 1133–1140. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Liu, X.; Geng, R.; Wang, M. Catalyst-free domino reaction of ethyl 4-hydroxyalkyl-2-ynoate with N-hetero-arylmethyl-N-2,2-difluoroethan-1-amine. Chin. J. Org. Chem. 2020, 40, 694–703. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, Y.; Xu, L.; Su, Y.; Jiang, J.; Wang, M. Synthesis and unexpected insecticidal activity of (E)-3-(1-iminoethyl)-5,5-disubstituted-4-(methylamino)furan-2(5H)-one. Chin. J. Org. Chem. 2020, 40, 2547–2554. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, B.; Guan, A.; Wang, W.; Zhang, Z.; Wang, M. Synthesis and fungicidal activity of (E)-5-[1-(4-phenyl-2-oxo-1-oxaspiro[4.5]dec-3-en-3-yl)ethylidene]-2-aminoimidazolin-4-one derivatives. Synthesis 2017, 49, 4663–4669. [Google Scholar]
- Guan, A.; Zhao, Y.; Wang, W.; Liu, X.; Wang, M. Synthesis and fungicidal activity of 3-acetyl-4-phenyl-1-oxaspiro[4,5]dec-3-en-2-one derivatives. Chin. J. Org. Chem. 2018, 38, 2767–2774. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xu, L.; Ma, H.; Li, X.; Wang, M. Synthesis and fungicidal activity of novel butenolide compounds containing oxime ether moiety. Chin. J. Org. Chem. 2022, 42, 2438–2448. [Google Scholar] [CrossRef]
- Kanako, I.; Tomohiro, T.; Hideo, I.; Pyoyun, P.; Kenichi, I. Cytological evaluation of the effect of azoxystrobin and alternative oxidase inhibitors in Botrytis cinerea. FEMS Microbiol. Lett. 2012, 326, 83–90. [Google Scholar]
- Esser, L.; Quinn, B.; Li, Y.-F.; Zhang, M.; Elberry, M.; Yu, L.; Yu, C.-A.; Xia, D. Crystallographic studies of quinol oxidation site inhibitors: A modified classification of inhibitors for the cytochrome bc1 complex. J. Mol. Biol. 2004, 341, 281–302. [Google Scholar] [CrossRef]
- Shivakumar, S.B.; Basavaraju, Y.B.; Umesha, B.; Krishna, M.H.; Mallesha, N.C. Synthesis and evaluation of antimitotic activity of new tetralone acid analogues of podophyllotoxin. Eur. J. Chem. 2014, 5, 424–429. [Google Scholar] [CrossRef]
- Zhou, L.; Gevorgyan, V. Double duty for cyanogen bromide in a cascade synthesis of cyanoepoxides. Angew. Chem. Int. Ed. 2011, 50, 2808–2810. [Google Scholar]







| Compds. | T. cucumeris | S. sclerotiorum | P. capsica | B. cinerea | F. graminearum |
|---|---|---|---|---|---|
| V-1 | 54.7 ± 4.0 | 7.2 ± 2.3 | 21.3 ± 1.0 | 45.8 ± 1.7 | 38.4 ± 2.9 |
| V-2 | 48.3 ± 3.1 | 63.7 ± 9.4 | 27.0 ± 1.0 | 31.3 ± 9.1 | 33.9 ± 1.5 |
| V-3 | 39.8 ± 0.9 | 9.3 ± 4.6 | 9.8 ± 1.0 | 12.9 ± 2.3 | 31.1 ± 1.5 |
| V-4 | 42.3 ± 0.9 | 40.0 ± 8.7 | 32.8 ± 3.0 | 24.4 ± 2.3 | 34.5 ± 0.1 |
| V-5 | 49.8 ± 2.3 | 32.0 ± 2.0 | 19.0 ± 3.0 | 45.8 ± 0.9 | 35.6 ± 2.5 |
| V-6 | 43.8 ± 1.3 | 79.4 ± 2.8 | 23.6 ± 1.2 | 46.2 ± 0.8 | 26.1 ± 0.9 |
| V-7 | 51.9 ± 1.2 | 80.6 ± 2.8 | 36.6 ± 3.5 | 28.3 ± 0.8 | 37.3 ± 2.5 |
| V-8 | 39.4 ± 4.3 | 31.5 ± 4.6 | 50.3 ± 1.3 | 27.2 ± 2.0 | 36.2 ± 3.1 |
| V-9 | 29.2 ± 1.6 | 9.7 ± 1.0 | 22.9 ± 2.9 | 7.1 ± 1.9 | 20.0 ± 2.3 |
| V-10 | 60.5 ± 2.1 | 89.1 ± 1.8 | 43.2 ± 1.8 | 25.0 ± 3.0 | 45.4 ± 2.4 |
| V-11 | 54.0 ± 1.6 | 55.8 ± 1.6 | 25.4 ± 2.2 | 19.5± 1.5 | 35.3 ± 2.0 |
| V-12 | 45.7 ± 3.2 | 45.8 ± 1.6 | 25.4 ± 1.2 | 27.2 ± 0.8 | 41.6 ± 0.1 |
| V-13 | 43.5 ± 2.4 | 47.2 ± 2.0 | 32.1 ± 0.8 | 27.6 ± 1.4 | 28.4 ± 0.4 |
| V-14 | 32.7 ±1.8 | 38.5 ± 1.5 | 31.8 ± 0.9 | 15.8 ±0.2 | 32.0 ± 0.6 |
| VI-1 | 48.8 ± 7.4 | 0.0 ± 2.8 | 35.6 ± 1.0 | 37.3 ± 2.6 | 54.8 ± 2.2 |
| VI-2 | 48.8 ± 1.7 | 41.6 ± 2.2 | 35.1 ± 1.0 | 34.3 ± 1.5 | 52.6 ± 0.9 |
| VI-3 | 47.8 ± 3.0 | 74.2 ± 2.2 | 17.8 ± 1.0 | 26.9± 1.5 | 46.3 ± 1.8 |
| VI-4 | 54.7 ± 2.3 | 63.4 ± 2.2 | 25.3 ± 1.0 | 34.3 ± 2.6 | 52.5 ± 0.9 |
| VI-5 | 51.2 ± 0.9 | 75.3 ± 1.5 | 29.9 ± 1.0 | 4.0 ± 1.7 | 37.3 ± 0.2 |
| VI-6 | 54.7 ± 1.6 | 71.0 ± 1.9 | 11.2 ± 1.3 | 8.1 ± 3.7 | 42.0 ± 1.8 |
| VI-7 | 50.8 ± 2.1 | 79.1 ± 0.1 | 28.4 ± 3.0 | 28.2 ± 2.9 | 37.3 ± 3.0 |
| VI-8 | 28.1 ± 1.6 | 0.0 ± 0.01 | 29.5 ± 2.8 | 7.1 ± 1.1 | 16.8 ± 0.9 |
| VI-9 | 69.2 ± 1.9 | 9.2 ± 0.2 | 44.2 ± 2.2 | 33.1 ± 1.3 | 42.7 ± 2.2 |
| VI-10 | 58.9 ± 2.1 | 84.8 ± 2.8 | 60.2 ± 4.3 | 31.0 ± 2.2 | 43.2 ± 3.4 |
| VI-11 | 38.5 ± 1.8 | 33.8 ± 1.4 | 22.8 ± 1.6 | 4.8 ± 3.2 | 32.3 ± 1.3 |
| VI-12 | 44.3 ± 0.4 | 55.9 ± 0.9 | 46.8 ± 1.0 | 37.1 ± 0.6 | 40.4 ± 0.7 |
| VI-13 | 42.0 ± 0.9 | 24.1 ± 1.3 | 16.6 ± 2.3 | 3.2 ± 0.1 | 17.9 ± 2.7 |
| VI-14 | 48.3 ± 0.4 | 41.7 ± 1.3 | 25.4 ±1.1 | 32.6 ± 0.4 | 38.6 ± 0.6 |
| 3–8 | 73.5 ± 0.8 | 81.9 ± 1.5 | 21.8 ± 1.6 | 53.5 ± 2.8 | 73.5 ± 0.8 |
| TRI | 69.8 ± 1.8 | 68.6 ± 0.6 | 2.0 ± 0.0 | 49.7 ± 0.5 | 43.9 ± 1.5 |
| Compds. | Regression Eq. | R2 | EC50(mg/L) (95%CL) |
|---|---|---|---|
| V-6 | y = 0.96x + 4.25 | 0.9890 | 1.51 (0.54~2.63) |
| V-7 | y = 1.09x + 4.45 | 0.9985 | 3.12 (0.97~5.27) |
| V-10 | y = 0.57x + 4.95 | 0.9918 | 3.06 (0.61~5.61) |
| VI-3 | y = 1.37x + 3.82 | 0.9830 | 7.13 (4.75~9.45) |
| VI-5 | y = 1.31x + 3.54 | 0.9816 | 13.24(9.94~16.64) |
| VI-6 | y = 1.44x + 2.71 | 0.9730 | 38.19 (25.32~51.06) |
| VI-7 | y = 1.37x + 4.90 | 0.9946 | 1.81 (0.01~4.77) |
| VI-10 | y = 1.10x + 4.23 | 0.9652 | 4.95(2.67~7.20) |
| 3–8 | y = 1.40x + 3.57 | 0.9710 | 10.62 (8.67~12.86) |
| TRI | y = 0.53x + 3.59 | 0.9970 | 38.09 (30.35~59.21) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, Y.; Zhao, B.; Xu, L.; Ma, H.; Wang, M. Synthesis and Antifungal Activity of New butenolide Containing Methoxyacrylate Scaffold. Molecules 2022, 27, 6541. https://doi.org/10.3390/molecules27196541
Zhang Q, Li Y, Zhao B, Xu L, Ma H, Wang M. Synthesis and Antifungal Activity of New butenolide Containing Methoxyacrylate Scaffold. Molecules. 2022; 27(19):6541. https://doi.org/10.3390/molecules27196541
Chicago/Turabian StyleZhang, Qian, Yihao Li, Bin Zhao, Leichuan Xu, Haoyun Ma, and Mingan Wang. 2022. "Synthesis and Antifungal Activity of New butenolide Containing Methoxyacrylate Scaffold" Molecules 27, no. 19: 6541. https://doi.org/10.3390/molecules27196541