Isolation, Structural Characterization, and Biological Activity of the Two Acidic Polysaccharides from the Fruits of the Elaeagnus angustifolia Linnaeus
Abstract
:1. Introduction
2. Results and Discussion
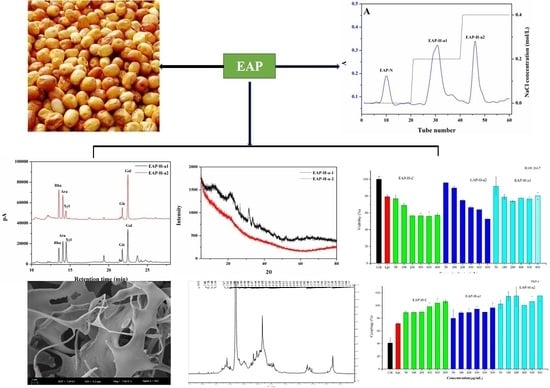
2.1. Chemical Composition of the Polysaccharides
2.2. Structural Characterization of the Polysaccharides
2.2.1. UV and FTIR Analysis
2.2.2. Conformational Analysis
2.2.3. Morphological Properties
2.2.4. X-ray Diffraction Analysis
2.2.5. Thermal Properties
2.2.6. NMR Results
2.3. Biological Activity
2.3.1. Antioxidant Activity
2.3.2. MTT Cell Viability
2.3.3. Effects of Polysaccharides on Macrophage Activation
2.3.4. Effect of EAP on Phagocytosis of Macrophages
3. Materials and Methods
3.1. Materials and Reagents
3.2. Pretreatment and Sequential Extraction of the Polysaccharides
3.3. Purification of the EAP-H
3.4. Structural Characterization
3.4.1. The Specific Optical Rotations
3.4.2. Monosaccharide Composition Analysis
3.4.3. Molecular Weight Determination and Homogeneity Analysis
3.4.4. UV and FTIR Spectroscopy Analysis
3.4.5. SEM Analysis
3.4.6. XRD Analysis
3.4.7. Analysis of Thermogravimetric and Differential Scanning Calorimetry
3.4.8. Congo Red Analysis
3.4.9. NMR Analysis
3.5. Antioxidant Activity
3.6. Cytotoxicity Assay
3.7. Assay of Macrophage Phagocytosis
3.8. Antiinflammatory Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Editorial Board of Flora of China of Chinese Academy of Sciences. Flora of China; Science Press: Peking, China, 1987. [Google Scholar]
- Xinjiang Institute of Biological Soil Desert Chinese Academy of Sciences. Flora of Medicinal Plants in Xinjiang; Volksverlag Xinjiang: Urumqi, China, 1984. [Google Scholar]
- Pharmacopoeia Committee of the Ministry of the Health of the People’s Republic of China. Drug Standards of the Ministry of Health of the People’s Republic of China Uygur Medicine Volume; Science/Technology and Health Publishing House of Xinjiang: Urumqi, China, 1999. [Google Scholar]
- Waili, A.; Yili, A.; Maksimov, V.V.; Mijiti, Y.; Atamuratov, F.N.; Ziyavitdinov, Z.F.; Mamadrakhimov, A.; Aisa, H.A.; Salikhov, S.I. Isolation of Biologically Active Constituents from Fruit of Elaeagnus angustifolia. Chem. Nat. Compd. 2016, 52, 574–576. [Google Scholar] [CrossRef]
- Wang, B.; Tai, M.; Zhang, K.; Chen, H.; Gan, X.; Che, B.; Abudukelimu, N.; Wang, G.; Xin, X.; Lin, L.; et al. Elaeagnus L gum polysaccharides alleviate the impairment of barrier function in the dry skin model mice. J. Cosmet. Dermatol. 2021, 20, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M.; Shahlari, N.; Hamidpour, R. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J. Tradit. Complement. Med. 2017, 7, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, J.; Tian, S.; Gu, H.; Li, N.; Sun, Y.; Ru, J.; Wang, J. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. pulp. Carbohydr. Polym. 2016, 151, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Sharifian-Nejad, M.S.; Shekarchizadeh, H. Physicochemical and functional properties of oleaster (Elaeagnus angustifolia L.) polysaccharides extracted under optimal conditions. Int. J. Biol. Macromol. 2019, 124, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Yang, J.F.; Liu, H. Purification and antioxidant activity of polysaccharide from Elaeagnus angustifolia in Xinjiang. Food Res. Dev. 2016, 37, 37–40. [Google Scholar]
- Yang, X.; Long, H.T.; Qiao, H.J. Optimization of preparation technology of oleaster polysaccharide effervescent tablets by response surface method. Sci. Technol. Food Ind. 2016, 37, 256–261. [Google Scholar]
- Shang, X.L.; Liu, C.Y.; Dong, H.Y.; Peng, H.H.; Zhu, Z.Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Qian, J.Y.; Chen, W.; Zhang, W.M.; Zhang, H. Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: A primary approach. Carbohydr. Polym. 2009, 78, 620–625. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, H.P.; Zhao, F.; Zhang, C.P.; Wang, Y.; Xu, J.J. Purification and preliminary analysis of Elaeagnus angustifolia L. polysaccharide-1a. Sci. Technol. Food Ind. 2015, 36, 138–142. [Google Scholar]
- Chen, Q.; Chen, J.; Du, H.; Li, Q.; Chen, J.; Zhang, G.; Liu, H.; Wang, J. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int. J. Mol. Sci. 2014, 15, 11446–11455. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; He, Z.G.; Li, X.Y.; Shen, W.D.; Wang, J.H.; Zhao, D.; Sun, H.; Xu, X.L.; Li, C.L.; Zha, X.Q. Chemical structure, antioxidant and anti-inflammatory activities of two novel pectin polysaccharides from purple passion fruit (Passiflora edulia Sims) peel. J. Mol. Struct. 2022, 1264, 133309. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Sun, W.; Kong, Y.; Jia, L. Antioxidation, anti-inflammation and anti-fibrosis effect of phosphorylated polysaccharides from Pleurotus djamor mycelia on adenine-induced chronic renal failure mice. Int. J. Biol. Macromol. 2021, 170, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qin, H.Y.; An, R.F.; Zhang, W.J.; Liu, J.X.; Yu, Q.F.; Liu, W.; Huang, X.F. Isolation, purification, structural characterization and antitumor activities of a polysaccharide from Lilium davidii var. unicolor Cotton. J. Mol. Struct. 2022, 1261, 132941. [Google Scholar] [CrossRef]
- Song, Q.Y.; Teng, A.G.; Zhu, Z.Y. Chemical structure and inhibition on α-glucosidase of a novel polysaccharide from Hypsizygus marmoreus. J. Mol. Struct. 2020, 1211, 128110. [Google Scholar] [CrossRef]
- Hu, T.G.; Zou, Y.X.; Li, E.N.; Liao, S.T.; Wu, H.; Wen, P. Effects of enzymatic hydrolysis on the structural, rheological, and functional properties of mulberry leaf polysaccharide. Food Chem. 2021, 355, 129608. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Wang, Q.; Lin, R.; He, P.; Lai, F.; Zhang, M.; Wu, H. Structural characterization and immunomodulatory activity of a novel acid polysaccharide isolated from the pulp of Rosa laevigata Michx fruit. Int. J. Biol. Macromol. 2020, 145, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhu, C.P.; Huang, G.Q.; Xiao, J.X. Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process Biochem. 2021, 109, 37–45. [Google Scholar] [CrossRef]
- Patel, M.K.; Tanna, B.; Mishra, A.; Jha, B. Physicochemical characterization, antioxidant and anti-proliferative activities of a polysaccharide extracted from psyllium (P. ovata) leaves. Int. J. Biol. Macromol. 2018, 118, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Suvakanta, D.; Narsimha, M.P.; Pulak, D.; Joshabir, C.; Biswajit, D. Optimization and characterization of purified polysaccharide from Musa sapientum L. as a pharmaceutical excipient. Food Chem. 2014, 149, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Al-Amoudi, R.H.; Taylan, O.; Kutlu, G.; Can, A.M.; Sagdic, O.; Dertli, E.; Yilmaz, M.T. Characterization of chemical, molecular, thermal and rheological properties of medlar pectin extracted at optimum conditions as determined by Box-Behnken and ANFIS models. Food Chem. 2019, 271, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, C.; Ran, C.; Tan, Y.; Yu, Q.; Kan, J. Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2019, 130, 903–914. [Google Scholar] [CrossRef]
- He, J.L.; Guo, H.; Wei, S.Y.; Zhou, J.; Xiang, P.Y.; Liu, L.; Zhao, L.; Qin, W.; Gan, R.Y.; Wu, D.T. Effects of different extraction methods on the structural properties and bioactivities of polysaccharides extracted from Qingke (Tibetan hulless barley). J. Cereal Sci. 2020, 92, 102906. [Google Scholar] [CrossRef]
- Shen, D.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Zhu, J.; Li, C.; Kou, X. Jujube peel polyphenols synergistically inhibit lipopolysaccharide-induced inflammation through multiple signaling pathways in RAW 264.7 cells. Food Chem. Toxicol. 2022, 164, 113062. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bai, Y.; Chen, G.; Dong, W.; Peng, Y.; Xu, W.; Sun, Y.; Zeng, X.; Liu, Z. Immunomodulatory activity of polysaccharides from the mycelium of Aspergillus cristatus, isolated from Fuzhuan brick tea, associated with the regulation of intestinal barrier function and gut microbiota. Food Res. Int. 2022, 152, 110901. [Google Scholar] [CrossRef]
- Wei, H.L.; Wang, Y.J.; Li, W.M.; Qiu, Y.; Hua, C.P.; Zhang, Y.B.; Guo, Z.H.; Xie, Z.K. Immunomodulatory activity and active mechanisms of a low molecular polysaccharide isolated from Lanzhou lily bulbs in RAW264.7 macrophages. J. Funct. Foods 2022, 92, 105071. [Google Scholar] [CrossRef]
- Zhu, L.J.; Li, W.; Fan, Z.Y.; Ye, X.Y.; Lin, R.Y.; Ban, M.M.; Ren, L.Z.; Chen, X.Q.; Zhang, D.Y. Immunomodulatory activity of polysaccharide from Arca granosa Linnaeus via TLR4/MyD88/NFκB and TLR4/TRIF signaling pathways. J. Funct. Foods 2021, 84, 104579. [Google Scholar] [CrossRef]
- Yang, L.H.; Liu, J.; Xia, X.W.; Wong, I.N.; Chung, S.K.; Xu, B.J.; El-Seedi, H.R.; Wang, B.; Huang, R.M. Sulfated heteropolysaccharides from Undaria pinnatifida: Structural characterization and transcript-metabolite profiling of immunostimulatory effects on RAW264.7 cells. Food Chem. X 2022, 13, 100251. [Google Scholar] [CrossRef]
- Yili, A.; Yimamu, H.; Ghulameden, S.; Hai Qing, Z.; Aisa, H.; Morlock, G. Determination of antidiabetic polysaccharides of Ocimum basilicum seeds indigenous to Xinjiang of China by high-performance thin-layer chromatography-UV/Vis-mass spectrometry. J. Planar Chromatogr. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Nuerxiati, R.; Mutailifu, P.; Gao, Y.H.; Lu, C.F.; Yili, A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crops Prod. 2022, 176, 114319. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.H.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nuerxiati, R.; Abuduwaili, A.; Mutailifu, P.; Wubulikasimu, A.; Rustamova, N.; Cui, J.X.; Aisa, H.A.; Yili, A. Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int. J. Biol. Macromol. 2019, 141, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Shang, H.M.; Guo, Y.; Zhang, H.X.; Wu, H.X. Comparison of different extraction methods of polysaccharides from cup plant (Silphium perfoliatum L.). Process Biochem. 2020, 90, 241–248. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Mutailifu, P.; Nuerxiati, R.; Gao, Y.H.; Aisa, H.A.; Yili, A. Structure and biological activity of polysaccharides from Nitraria sibirica pall fruit. Food Biosci. 2021, 40, 100903. [Google Scholar] [CrossRef]













| EAP-H-a1 | EAP-H-a2 | |
|---|---|---|
| Neutral sugar (%) | 12.55 | 12.24 |
| Protein (%) | 0 | 0 |
| Uronic acid (%) | 67.15 | 62.24 |
| Mw (kDa) | 705.79 | 439.85 |
| [α]20 D (c 1.0 mg/mL, H2O) | +290.2 | +175.9 |
| Monosaccharide Composition (Molar Ratio) | ||
| Rha | 13.752 | 24.861 |
| Ara | 20.574 | 19.746 |
| Xyl | 23.361 | 8.282 |
| Glc | 8.894 | 8.474 |
| Gal | 33.418 | 38.637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huojiaaihemaiti, H.; Mutaillifu, P.; Omer, A.; Nuerxiati, R.; Duan, X.; Xin, X.; Yili, A. Isolation, Structural Characterization, and Biological Activity of the Two Acidic Polysaccharides from the Fruits of the Elaeagnus angustifolia Linnaeus. Molecules 2022, 27, 6415. https://doi.org/10.3390/molecules27196415
Huojiaaihemaiti H, Mutaillifu P, Omer A, Nuerxiati R, Duan X, Xin X, Yili A. Isolation, Structural Characterization, and Biological Activity of the Two Acidic Polysaccharides from the Fruits of the Elaeagnus angustifolia Linnaeus. Molecules. 2022; 27(19):6415. https://doi.org/10.3390/molecules27196415
Chicago/Turabian StyleHuojiaaihemaiti, Haibaier, Paiheerding Mutaillifu, Adil Omer, Rehebati Nuerxiati, Xiaomei Duan, Xuelei Xin, and Abulimiti Yili. 2022. "Isolation, Structural Characterization, and Biological Activity of the Two Acidic Polysaccharides from the Fruits of the Elaeagnus angustifolia Linnaeus" Molecules 27, no. 19: 6415. https://doi.org/10.3390/molecules27196415