Inositol Derivatives with Anti-Inflammatory Activity from Leaves of Solanum capsicoides Allioni
Abstract
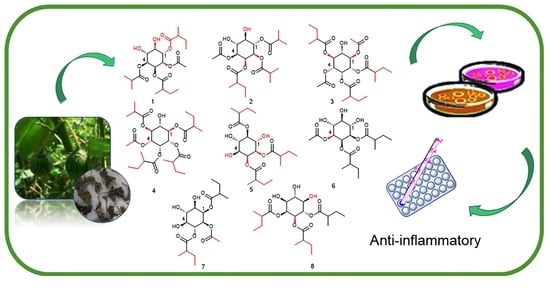
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Bioactive Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siracusa, L.; Napoli, E.; Ruberto, G. Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules 2022, 27, 1525. [Google Scholar] [CrossRef] [PubMed]
- Michell, R.H. Inositol derivatives: Evolution and functions. Nat. Rev. Mol. Cell. Biol. 2008, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Krakowska, A.; Górecki, R.; Buszewski, B. Simultaneous Determination of Cyclitols and Sugars Following a Comprehensive Investigation of 40 Plants. Food Anal. Methods 2019, 12, 1466–1478. [Google Scholar] [CrossRef]
- Papaleo, E.; Unfer, V.; Baillargeon, J.P.; Chiu, T.T. Contribution of myo-inositol to reproduction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Simões, L.O.; Conceição-Filho, G.; Ribeiro, T.S.; Jesus, A.M.; Fregoneze, J.B.; Silva, A.Q.G.; Petreanu, M.; Cechinel-Filho, V.; Niero, R.; Niero, H.; et al. Evidences of antihypertensive potential of extract from Solanum capsicoides All. in spontaneously hypertensive rats. Phytomedicine 2016, 23, 498–508. [Google Scholar] [CrossRef]
- Petreanu, M.; Maia, P.; da Rocha Pittarello, J.L.; Loch, L.C.; Monache, F.D.; Perez, A.L.; Solano-Arias, G.; Filho, V.C.; de Souza, M.M.; Niero, R. Antidepressant-like effect and toxicological parameters of extract and withanolides isolated from aerial parts of Solanum capsicoides All. (Solanaceae). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 979–990. [Google Scholar] [CrossRef]
- Huque, A.; Biswas, S.; Abdullah-Al-Mamun, M.; Bhuiyan, J.R.; ur Rashid, M.H.; Jahan, A. Analgesic, anti-inflammatory and anxiolytic activity evaluation of methanolic extract of Solanum surattense leaf in Swiss Albino mice model. Int. J. Pharm. Clin. Res. 2015, 7, 68–76. [Google Scholar]
- Vijay Amirtharaj, L.; Srinivasan, N.; Sireesha Abburi Karthikeyan, K.; Mahalaxmi, S. Evaluating the Analgesic Efficacy of Solanum surattense (Herbal Seed Extract) in Relieving Pulpal Pain-An In-vivo Study. Dentistry 2015, 5, 4. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chen, Y.-Y.; Lin, Y.-C.; Huang, C.-Y.; Uvarani, C.; Hwang, T.-L.; Chiang, M.Y.; Liu, H.-Y.; Sheu, J.-H. Capsisteroids A–F, withanolides from the leaves of Solanum Capsicoides. RSC Adv. 2015, 5, 88841–88847. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, J.; Kong, L. Steroidal alkaloid saponins and steroidal saponins from Solanum Surattense. Phytochemistry 2011, 72, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Ahmed, E.; Sharif, A.; Arshad, M.; Batool, N.; Rasool, M.A.; Mukhtar Ul, H. Two New Steroidal Glycosides from Solanum surattense. Chem. Nat. Compd. 2014, 49, 1091–1094. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Jang, Y.-K.; Wang, S.-Y.; Li, X.-M.; Pan, J.; Guan, W.; Algradi, A.M.; Kuang, H.-X.; Yang, B.-Y. Phenylpropanoids from Solanum capsicoides and their anti-inflammatory activity. J. Asian Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Jie-Hui, L.I.; Yin, H.L.; Dong, J.X. Phenylpropanoids from Solanum Surattense. Mil. Med. Sci. 2013, 37, 130–134. [Google Scholar] [CrossRef]
- Kong, J.; Du, Z.H.; Dong, L. Pinitol Prevents Lipopolysaccharide (LPS)-Induced Inflammatory Responses in BV2 Microglia Mediated by TREM2. Neurotox. Res. 2020, 38, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Lassila, M.; Jarvenpaa, E.; Haraldsson, G.G.; Jonsdottir, S.; Yang, B. Inositols and methylinositols in sea buckthorn (Hippophaë rhamnoides) berries. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 1426–1432. [Google Scholar] [CrossRef]
- Abreu, P.; Relva, A. Carbohydrates from Detarium microcarpum bark extract. Carbohydr. Res. 2002, 337, 1663–1666. [Google Scholar] [CrossRef]
- Wu, J.W.; Tang, C.P.; Yao, S.; Zhang, L.; Ke, C.Q.; Feng, L.; Lin, G.; Ye, Y. Anti-inflammatory Inositol Derivatives from the Whole Plant of Inula cappa. J. Nat. Prod. 2015, 78, 2332–2338. [Google Scholar] [CrossRef]
- Garayev, E.; Herbette, G.; Di Giorgio, C.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. New sesquiterpene acid and inositol derivatives from Inula montana L. Fitoterapia 2017, 120, 79–84. [Google Scholar] [CrossRef]
- Fortuna, A.M.; Juarez, Z.N.; Bach, H.; Nematallah, A.; Av-Gay, Y.; Sanchez-Arreola, E.; Catalan, C.A.; Turbay, S.; Hernandez, L.R. Antimicrobial activities of sesquiterpene lactones and inositol derivatives from Hymenoxys Robusta. Phytochemistry 2011, 72, 2413–2418. [Google Scholar] [CrossRef]
- Gao, F.; Wang, H.P.; Mabry, T.J. Sesquiterpene lactone aglycones and glycosides and inositol derivatives from Hymenoxys biennis. Phytochemistry 1990, 29, 3875–3880. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Ho, D.V.; Nguyen, H.M.; Do, T.T.; Phan, K.V.; Morita, H.; Heinamaki, J.; Raal, A.; Nguyen, H.T. Chiro-Inositol Derivatives from Chisocheton paniculatus Showing Inhibition of Nitric Oxide Production. J. Nat. Prod. 2020, 83, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Tran, L.T.T.; Ho, D.V.; Phan, K.V.; Raal, A.; Morita, H. Three new inositol derivatives from Chisocheton paniculatus. Tetrahedron Lett. 2019, 60, 1841–1844. [Google Scholar] [CrossRef]
- Gambioli, R.; Montanino Oliva, M.; Nordio, M.; Chiefari, A.; Puliani, G.; Unfer, V. New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines 2021, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Colodny, L.; Hoffman, R.L. Inositol—Clinical applications for exogenous use. Altern. Med. Rev. 1998, 3, 432–447. [Google Scholar] [PubMed]
- Fux, M.; Levine, J.; Aviv, A.; Belmaker, R.H. Inositol treatment of obsessive-compulsive disorder. Am. J. Psychiatry 1996, 153, 1219–1221. [Google Scholar] [CrossRef]
- Raju, K.; Anbuganapathi, G.; Gokulakrishnan, V.; Rajkapoor, B.; Jayakar, B.; Manian, S. Effect of Dried Fruits of Solanum nigrum LINN against CCl4-Induced Hepatic Damage in Rats. Biol. Pharm. Bull. 2003, 26, 1618–1619. [Google Scholar] [CrossRef]
- Jarald, E.E.; Edwin, S.; Saini, V.; Deb, L.; Gupta, V.B.; Wate, S.P.; Busari, K.P. Anti-inflammatory and anthelmintic activities of Solanum khasianum Clarke. Nat. Prod. Res. 2008, 22, 269–274. [Google Scholar] [CrossRef]
- Ndebia, E.J.; Kamgang, R.; Nkeh-ChungagAnye, B.N. Analgesic and Anti-Inflammatory Properties of Aqueous Extract from Leaves of Solanum Torvum (Solanaceae). Afr. J. Trad. CAM 2007, 4, 240–244. [Google Scholar] [CrossRef] [Green Version]






| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.34 (t, 10.0) | 5.33 (t, 10.0) | 5.41 (td, 10.0, 5.2) | 5.43 (t, 10.0) | 3.85 (t, 10.1) | 3.78 (t, 9.7) | 5.30 (t, 10.0) | 3.44 (t, 10.0) |
| 2 | 5.06 (dd, 10.0, 2.8) | 5.08 (dd, 10.0, 2.8) | 5.15 (dt, 10.0, 2.8) | 5.16 (ddd, 13.4, 10.0, 2.8) | 4.83 (overlap) | 4.83 (overlap) | 4.92 (dd, 10.0, 2.8) | 5.50 (t, 2.8) |
| 3 | 5.56 (t, 2.8) | 5.55 (t, 2.8) | 5.60 (t, 2.8) | 5.60 (t, 2.8) | 5.51 (t, 2.5) | 5.53 (t, 2.8) | 5.48 (t, 2.8) | 4.96 (dd, 10.0, 2.8) |
| 4 | 4.89 (dd, 10.0, 2.8) | 4.89 (overlap) | 5.15 (dt, 10.0, 2.8) | 5.16 (ddd, 13.4, 10.0, 2.8) | 3.69 (overlap) | 4.81 (overlap) | 3.44 (t, 10.0) | 5.32 (t, 10.0) |
| 5 | 3.84 (t, 10.0) | 3.84 (t, 10.0) | 5.41 (td, 10.0, 5.2) | 5.38 (t, 10.0) | 3.69 (overlap) | 3.75 (t, 9.7) | 3.67 (overlap) | 3.66 (overlap) |
| 6 | 3.54 (t, 10.0) | 3.56 (t, 10.0) | 3.79 (t, 10.0) | 3.79 (t, 10.0) | 4.88 (overlap) | 3.36 (t, 9.7) | 3.67 (overlap) | 3.66 (overlap) |
| 2MB-2 | 2.41 (q, 7.0) | 2.51 (m) | 2.27 (m) | 2.39 (m) | 2.33 (m) | 2.36 (m) | 2.52 (m) | 2.37 (q, 7.0) |
| 2MB-3 | 1.46 (m) 1.64 (m) | 1.71 (m) | 1.43 (m) 1.61(m) | 1.47 (m) 1.68 (m) | 1.41 (m) 1.70 (overlap) | 1.42 (m) 1.63 (m) | 1.60 (overlap) 1.74 (m) | 1.46 (m) 1.67 (m) |
| 2MB-4 | 0.89 (t, 7.4) | 0.98 (t, 7.4) | 0.84 (t, 7.4) | 0.92 (t, 7.4) | 0.96 (t, 7.4) | 0.85 (t, 7.4) | 0.99 (t, 7.4) | 0.92 (t, 7.5) |
| 2MB-5 | 1.12 (dd, 12.2, 7.0) | 1.20 (d, 7.0) | 1.05 (d, 7.0) | 1.10 (d, 7.0) | 1.18 (d, 7.0) | 1.11 (d, 7.0) | 1.17 (d, 7.0) | 1.21 (d, 7.0) |
| 2MB-2′ | 2.52 (overlap) | 2.55 (m) | 2.26 (m) | 2.46 (m) | 2.45 (m) | 2.41 (m) | 2.22 (q, 7.0) | |
| 2MB-3′ | 1.57 (m) 1.72 (m) | 1.61 (m) 1.75 (m) | 1.39 (m) 1.59 (m) | 1.51 (m) 1.70 (overlap) | 1.53 (m) 1.70 (m) | 1.45 (m) 1.60 (overlap) | 1.37 (m) 1.61 (overlap) | |
| 2MB-4′ | 0.98 (t, 7.4) | 1.00 (t, 7.4) | 0.83 (t, 7.4) | 0.96 (t, 7.4) | 0.97 (t, 7.4) | 0.88 (t, 7.4) | 0.86 (t, 7.4) | |
| 2MB-5′ | 1.20 (d, 7.0) | 1.22 (d, 7.0) | 1.04 (d, 7.0) | 1.18 (d, 7.0) | 1.17 (d, 7.0) | 1.12 (d, 7.0) | 1.04 (d, 7.0) | |
| 2MB-2″ | 2.41 (m) | 2.56 (m) | 2.46 (m) | 2.48 (q, 7.0) | ||||
| 2MB-3″ | 1.43 (m) 1.61 (m) | 1.59 (m) 1.76 (m) | 1.51 (m) 1.70 (overlap) | 1.52 (m) 1.74 (overlap) | ||||
| 2MB-4″ | 0.89 (t, 7.4) | 1.01 (t, 7.4) | 0.95 (t, 7.4) | 0.94 (t, 7.4) | ||||
| 2MB-5″ | 1.13 (d, 7.0) | 1.22 (d, 7.0) | 1.18 (d, 7.0) | 1.10 (d, 7.0) | ||||
| Ac-2 | 1.90 (s) | 2.00 (s) | 2.04 (s) | 1.91 (s) | 1.99 (s) | 1.89 (s) | ||
| Ac-2′ | 1.91 (s) | |||||||
| iBu-2 | 2.52 (overlap) | 2.56 (m) | 2.56 (m) | |||||
| iBu-3 | 1.13 (d, 7.0) | 1.12 (d, 7.0) | 1.13 (t, 7.1) | |||||
| iBu-4 | 1.13 (d, 7.0) | 1.15 (d, 7.0) | 1.13 (t, 7.1) | |||||
| iBu-2′ | 2.41 (m) | |||||||
| iBu-3′ | 1.06 (dd, 7.0, 1.2) | |||||||
| iBu-4′ | 1.06 (dd, 7.0, 1.2) |
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 72.5 | 72.8 | 73.3 | 72.7 | 70.6 | 72.1 | 72.3 | 74.2 |
| 2 | 71.0 | 70.6 | 70.2 | 70.4 | 73.3 | 72.4 | 71.7 | 73.0 |
| 3 | 69.8 | 69.8 | 69.6 | 69.7 | 72.4 | 69.9 | 72.6 | 71.5 |
| 4 | 72.3 | 72.7 | 70.8 | 70.8 | 71.1 | 73.1 | 73.9 | 72.5 |
| 5 | 72.3 | 72.2 | 72.3 | 72.8 | 72.6 | 72.0 | 74.5 | 74.6 |
| 6 | 73.9 | 73.7 | 71.6 | 71.6 | 76.6 | 75.9 | 70.6 | 70.9 |
| 2MB-1 | 177.5 | 177.2 | 176.6 | 177.2 | 177.6 | 177.6 | 178.2 | 177.5 |
| 2MB-2 | 42.5 | 42.6 | 42.4 | 42.2 | 42.4 | 42.2 | 42.4 | 42.2 |
| 2MB-3 | 27.8 | 27.9 | 27.5 | 27.7 | 27.5 | 27.7 | 28.0 | 27.7 |
| 2MB-4 | 11.9 | 12.0 | 12.0 | 11.8 | 11.9 | 11.8 | 11.8 | 11.8 |
| 2MB-5 | 17.0 | 17.4 | 17.0 | 17.0 | 17.0 | 16.8 | 16.8 | 17.4 |
| 2MB-1′ | 177.2 | 177.0 | 176.6 | 177.4 | 177.4 | 177.9 | 176.9 | |
| 2MB-2′ | 42.6 | 42.6 | 42.1 | 42.5 | 42.6 | 42.3 | 42.2 | |
| 2MB-3′ | 27.9 | 27.9 | 27.5 | 27.7 | 27.9 | 27.8 | 27.3 | |
| 2MB-4′ | 11.9 | 12.0 | 11.8 | 11.9 | 12.0 | 11.8 | 11.8 | |
| 2MB-5′ | 17.3 | 17.4 | 16.7 | 16.9 | 17.4 | 16.9 | 16.7 | |
| 2MB-1″ | 177.2 | 177.0 | 178.2 | 177.3 | ||||
| 2MB-2″ | 42.5 | 42.6 | 42.5 | 42.6 | ||||
| 2MB-3″ | 27.8 | 27.9 | 27.9 | 27.7 | ||||
| 2MB-4″ | 11.9 | 12.0 | 11.9 | 12.0 | ||||
| 2MB-5″ | 17.1 | 17.3 | 17.4 | 17.0 | ||||
| Ac-1 | 171.2 | 171.9 | 171.7 | 171.1 | 172.1 | 171.8 | ||
| Ac-2 | 20.6 | 20.7 | 20.9 | 20.5 | 20.8 | 20.8 | ||
| Ac-1′ | 171.1 | |||||||
| Ac-2′ | 20.6 | |||||||
| iBu-1 | 177.8 | 177.9 | 177.8 | |||||
| iBu-2 | 35.1 | 35.2 | 35.2 | |||||
| iBu-3 | 19.1 | 19.2 | 19.0 | |||||
| iBu-4 | 19.3 | 19.5 | 19.6 | |||||
| iBu-1′ | 177.1 | |||||||
| iBu-2′ | 35.1 | |||||||
| iBu-3′ | 19.0 | |||||||
| iBu-4′ | 19.1 |
| No. | IC50 (µM) | No. | IC50 (µM) |
|---|---|---|---|
| 1 | >100 | 5 | >100 |
| 2 | >100 | 6 | >100 |
| 3 | >100 | 7 | >100 |
| 4 | >100 | 8 | >100 |
| No. | IC50 (µM) | No. | IC50 (µM) |
|---|---|---|---|
| 1 | 24.27 ± 1.82 | 6 | 23.31 ± 0.74 |
| 2 | 31.66 ± 2.71 | 7 | 14.50 ± 1.22 |
| 3 | >100 | 8 | 31.03 ± 0.92 |
| 4 | >100 | NMMA | 1.67 ± 0.24 |
| 5 | 11.21 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Meng, X.; Wang, H.; Sun, Y.; Wang, S.-Y.; Jiang, Y.-K.; Algradi, A.M.; Naseem, A.; Kuang, H.-X.; Yang, B.-Y. Inositol Derivatives with Anti-Inflammatory Activity from Leaves of Solanum capsicoides Allioni. Molecules 2022, 27, 6063. https://doi.org/10.3390/molecules27186063
Liu Y, Meng X, Wang H, Sun Y, Wang S-Y, Jiang Y-K, Algradi AM, Naseem A, Kuang H-X, Yang B-Y. Inositol Derivatives with Anti-Inflammatory Activity from Leaves of Solanum capsicoides Allioni. Molecules. 2022; 27(18):6063. https://doi.org/10.3390/molecules27186063
Chicago/Turabian StyleLiu, Yan, Xin Meng, Han Wang, Yan Sun, Si-Yi Wang, Yi-Kai Jiang, Adnan Mohammed Algradi, Anam Naseem, Hai-Xue Kuang, and Bing-You Yang. 2022. "Inositol Derivatives with Anti-Inflammatory Activity from Leaves of Solanum capsicoides Allioni" Molecules 27, no. 18: 6063. https://doi.org/10.3390/molecules27186063