An Insight into Symmetrical Cyanine Dyes as Promising Selective Antiproliferative Agents in Caco-2 Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterisation
2.2. In Vitro Studies
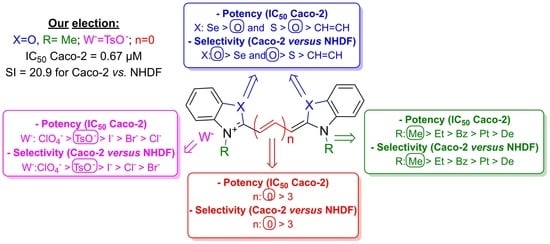
2.2.1. Antiproliferative Effects in Human Cell Lines
2.2.2. Photocytotoxicity and Photostability Evaluation
2.2.3. Morphological Analysis of Caco-2 Cells
2.2.4. Flow Cytometry Studies of Apoptosis and Cell Cycle Effects
2.3. Computational Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of bis(benzo[d]thiazol-2-yl)methane (1)
3.1.2. General Procedures (GP) for the Synthesis of Monomethine Cyanine Dyes 2–14
- Methyl-2-((3-methylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium chloride (2): using GP1, from 2-(methylthio)benzothiazole (181 mg), 2-methylbenzothiazole (149 mg), and methyl tosylate (409 mg). Anion exchange was caused by reflux in a hydrochloric acid solution 18.5% (10 mL) or a saturated sodium chloride solution (10 mL). Yield: 68%; yellow crystals; m.p. 265–266 °C (lit. 269 °C [32]); vis λmax (EtOH): 424 nm, log ε = 4.97; IR λmax (KBr): 3396, 1532, 1470, 1358, 1281, 756 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.20 (dd, J = 8.0, 1.2 Hz, 2H), 7.86 (d, J = 8.3 Hz, 2H), 7.66 (ddd, J = 8.1, 7.4, 1.0 Hz, 2H), 7.48 (t, J = 7.6 Hz, 2H), 6.70 (s, 1H), 4.02 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 162.1, 140.8, 128.6, 125.0, 124.8, 123.5, 113.9, 83.0, 34.2.
- 3-Methyl-2-((3-methylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium bromide (3): using GP1, from 2-(methylthio)benzothiazole (181 mg), 2-methylbenzothiazole (149 mg), and methyl tosylate (409 mg). Anion exchange was caused by reflux in a saturated sodium bromide solution (10 mL). Yield: 69%; yellow crystals; m.p. 302–303 °C (lit. 292–293 [40]); vis λmax (EtOH): 424 nm, log ε = 4.83; IR λmax (KBr): 3396, 1524, 1470, 1354, 1281, 756 cm−1. 1H NMR (400 MHz, DMSO-d6): δ 8.22 (dd, J = 8.0, 1.1 Hz, 2H), 7.88 (d, J = 8.3 Hz, 2H), 7.68 (ddd, J = 8.1, 7.3, 1.1 Hz, 2H), 7.50 (t, J = 7.7 Hz, 2H), 6.72 (s, 1H), 4.03 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 162.1, 140.8, 128.5, 124.9, 124.8, 123.5, 113.8, 82.9, 34.2.
- 3-Methyl-2-((3-methylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (4): using GP1, from 2-(methylthio)benzothiazole (181 mg), 2-methylbenzothiazole (149 mg), and methyl tosylate (409 mg). Anion exchange was caused by reflux in a saturated potassium iodide solution (10 mL). Yield: 94%; yellow crystals; m.p. 315–316 °C (lit. 303 °C [33]); vis λmax (EtOH): 424 nm, log ε = 4.96; IR λmax (KBr): 3438, 1524, 1477, 1358, 1285, 758 cm−1. 1H NMR (400 MHz, DMSO-d6): δ 8.21 (dd, J = 8.0, 1.2 Hz, 2H), 7.87 (d, J = 8.3 Hz, 2H), 7.67 (ddd, J = 8.4, 7.3, 1.2 Hz, 2H), 7.49 (ddd, J = 8.2, 7.3, 1.0 Hz, 2H), 6.70 (s, 1H), 4.02 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 162.1, 140.8, 128.5, 124.9, 124.8, 123.5, 113.9, 82.9, 34.1.
- 3-Methyl-2-((3-methylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium 4-methylbenzenesulfonate (5): using GP1, from 2-(methylthio)benzothiazole (181 mg), 2-methylbenzothiazole (149 mg), and methyl tosylate (409 mg). Yield: 77%; yellow crystals; m.p. 296–297 °C; vis λmax (EtOH): 424 nm, log ε = 4.98; IR λmax (KBr): 3384, 1632, 1536, 1474, 1362, 1282, 752 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.21 (dd, J = 8.1, 1.2 Hz, 2H), 7.88 (d, J = 8.3 Hz, 2H), 7.69 (ddd, J = 8.4, 7.3, 1.2 Hz, 2H), 7.50 (t, J = 7.3 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 6.72 (s, 1H), 4.03 (s, 6H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6): 162.1, 145.8, 140.8, 137.5, 128.5, 128.0, 125.5, 124.9, 124.8, 123.5, 113.8, 82.9, 34.1, 20.8.
- 3-Methyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium 4-methylbenzenesulfonate (6): using GP1, from 2-(ethylthio)benzothiazole (195 mg), 2-methylbenzothiazole (149 mg), and ethyl tosylate (441 mg). Yield: 80%; yellow crystals; m.p. 194–196 °C; vis λmax (EtOH): 425 nm, log ε = 4.84; IR λmax (KBr): 3068, 2997, 2923, 1529, 1465, 1373, 1339, 1269, 1192, 1128, 1034, 1011, 732, 681, 566 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.23 (dd, J = 8.0, 1.2 Hz, 2H), 7.90 (d, J = 8.3 Hz, 2H), 7.70 (dt, J = 7.7, 1.1 Hz, 2H), 7.51 (t, J = 7.6 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 7.9 Hz, 2H), 6.75 (s, 1H), 4.70 (q, J = 7.1 Hz, 4H), 2.28 (s, 3H), 1.38 (t, J = 7.1 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 161.3, 145.8, 139.7, 137.6, 128.6, 128.0, 125.5, 125.0, 124.9, 123.5, 113.6, 81.9, 41.6, 20.8, 12.3.
- 3-Ethyl-2-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium perchlorate (7): using GP1, from 2-(ethylthio)benzothiazole (195 mg), 2-methylbenzothiazole (149 mg), and ethyl tosylate (441 mg). Anion exchange was caused by reflux in a saturated sodium perchlorate solution (10 mL). Yield: 83%; yellow crystals; m.p. 296–297 °C; vis λmax (EtOH): 425 nm, log ε = 4.92; IR λmax (KBr): 3093, 2976, 2933, 1592, 1529, 1466, 1375, 1337, 1315, 1266, 1234, 1081, 747, 622 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.23 (dd, J = 8.1, 1.2 Hz, 2H), 7.91 (d, J = 8.3 Hz, 2H), 7.70 (ddd, J = 8.4, 7.2, 1.3 Hz, 2H), 7.51 (t, J = 7.7 Hz, 2H), 6.75 (s, 1H), 4.70 (q, J = 7.2 Hz, 4H), 1.38 (t, J = 7.0 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 161.4, 139.7, 128.6, 125.0, 124.9, 123.5, 113.6, 81.9, 41.6, 12.3.
- 3-Benzyl-2-((3-benzylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (8): using GP2, from 3-benzyl-2-methylbenzo[d]thiazol-3-ium iodide (367 mg). Yield: 25%; yellow crystals; m.p. 249–250 °C; vis λmax (EtOH): 427 nm, log ε = 4.87; IR λmax (KBr): 3019, 1562, 1471, 1407, 1355, 1309, 1282, 1218, 1159, 1131, 1025, 936, 819, 754 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.28 (dd, J = 8.0, 1.2 Hz, 2H), 7.87 (d, J = 8.3 Hz, 2H), 7.66 (ddd, J = 8.5, 7.3, 1.3 Hz, 2H), 7.52 (t, J = 7.6 Hz, 2H), 7.33–7.17 (m, 6H), 7.14 (dd, J = 8.0, 1.4 Hz, 4H), 6.96 (s, 1H), 5.91 (s, 4H); 13C NMR (101 MHz, DMSO-d6): δ 162.6, 140.4, 134.1, 129.0, 128.8, 127.9, 126.7, 125.3, 125.0, 123.8, 114.1, 83.4, 49.1; ESI-HRMS calcd for [M-I]+ C29H23N2S2+ 463.1297, found 463.1289.
- 3-(2-Methoxy-2-oxoethyl)-2-((3-(2-methoxy-2-oxoethyl)benzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium bromide (9): using GP2, from 3-(2-methoxy-2-oxoethyl)-2-methylbenzo[d]thiazol-3-ium bromide (302 mg). Yield: 28%; yellow crystals; m.p. 226–227 °C; vis λmax (EtOH): 427 nm, log ε = 5.13; IR λmax (KBr): 3373, 3075, 2990, 2851, 1737, 1501, 1360, 1290, 1009, 844, 759, 520 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.26 (d, J = 8.0 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.68 (t, J = 7.8 Hz, 2H), 7.54 (t, J = 7.7 Hz, 2H), 6.75 (s, 1H), 5.71 (s, 4H), 3.76 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 166.9, 163.4, 140.2, 128.9, 125.5, 124.6, 123.8, 113.8, 83.4, 53.0, 47.5; ESI-HRMS calcd for [M-Br]+ C21H19N2O4S2+ 427.0781, found 427.0770.
- 3-Pentyl-2-((3-pentylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (10): using GP2, from 2-methyl-3-pentylbenzo[d]thiazol-3-ium iodide (347 mg). Yield: 75%; yellow crystals; m.p. 164–166 °C; vis λmax (EtOH): 426 nm, log ε = 4.77; IR λmax (KBr): 3063, 2950, 2923, 2858, 1519, 1504, 1463, 1371, 1344, 1264, 1192, 746, 501 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (d, J = 7.9 Hz, 2H), 7.91 (d, J = 8.4 Hz, 2H), 7.69 (t, J = 7.8 Hz, 2H), 7.51 (t, J = 7.6 Hz, 2H), 6.68 (s, 1H), 4.65 (t, J = 7.5 Hz, 4H), 1.79 (p, J = 7.6 Hz, 4H), 1.44 (p, J = 7.0 Hz, 4H), 1.35 (sx, J = 7.1 Hz, 4H), 0.88 (t, J = 7.1 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 161.6, 140.2, 128.6, 125.0, 125.0, 123.6, 113.9, 82.5, 46.2, 28.2, 26.8, 22.0, 13.8; ESI-HRMS calcd for [M-I]+ C25H31N2S2+ 423.1923, found 423.1914.
- 3-Decyl-2-((3-decylbenzo[d]thiazol-2(3H)-ylidene)methyl)benzo[d]thiazol-3-ium iodide (11): using GP2, from 3-decyl-2-methylbenzo[d]thiazol-3-ium iodide (417 mg). Yield: 48%; yellow crystals; m.p. 232–233 °C; vis λmax (EtOH): 427 nm, log ε = 4.92; IR λmax (KBr): 3070, 3002, 2920, 2851, 1504, 1465, 1337, 1263, 771, 735, 523 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (d, J = 8.0 Hz, 2H), 7.91 (d, J = 8.4 Hz, 2H), 7.69 (t, J = 7.5 Hz, 2H), 7.51 (t, J = 7.7 Hz, 2H), 6.68 (s, 1H), 4.65 (t, J = 7.4 Hz, 4H), 1.77 (p, J = 7.3 Hz, 4H), 1.44 (p, J = 8.0 Hz, 4H), 1.30 (p, J = 7.1 Hz, 4H), 1.27–1.15 (m, 20H), 0.81 (t, J = 6.6 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 161.6, 140.2, 128.6, 125.0, 124.9, 123.6, 113.9, 82.6, 46.2, 31.3, 29.0, 29.0, 28.7, 27.1, 26.1, 22.1, 13.9; ESI-HRMS calcd for [M-I]+ C35H51N2S2+ 563.3488, found 563.3476.
- 3-Methyl-2-((3-methylbenzo[d]oxazol-2(3H)-ylidene)methyl)benzo[d]oxazol-3-ium 4-methylbenzenesulfonate (12): using GP1, from 2-(methylthio)benzoxazole (165 mg), 2-methylbenzoxazole (133 mg), and methyl tosylate (409 mg). Yield: 66%; yellowish white crystals; m.p. 297–298 °C; vis λmax (EtOH): 376 nm, log ε = 4.96; IR λmax (KBr): 3017, 1605, 1597, 1489, 1331, 1220, 1100, 752 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 7.83 (d, J = 8.0 Hz, 2H), 7.70 (dd, J = 8.0, 1.2 Hz, 2H), 7.53 (td, J = 7.8, 1.1 Hz, 2H), 7.50–7.40 (m, 4H), 7.10 (d, J = 7.8 Hz, 2H), 5.80 (s, 1H), 3.83 (s, 6H), 2.28 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δ 161.8, 146.2, 145.8, 137.5, 131.2, 128.0, 126.0, 125.5, 124.9, 111.3, 111.0, 57.5, 30.7, 20.8.
- 1-Methyl-2-((1-methylquinolin-2(1H)-ylidene)methyl)quinolin-1-ium iodide (13): using GP3, from 1,2-dimethylquinolin-1-ium 4-methylbenzenesulfonate (329 mg), 1-methyl-2-(methylthio)quinolin-1-ium 4-methylbenzenesulfonate (361 mg) in ethanol. Anion exchange was caused by reflux in a saturated potassium iodide solution (10 mL). Yield: 76%; brown powder; m.p. 240–241 °C (lit. 245–246 °C [34]); vis λmax (EtOH): 523 nm, log ε = 4.60; IR λmax (KBr): 3452, 2981, 1600, 1500, 1328, 1224, 752 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.14 (d, J = 9.3 Hz, 2H), 8.00 (d, J = 8.7 Hz, 2H), 7.93 (dd, J = 7.9, 1.5 Hz, 2H), 7.83 (ddd, J = 8.7, 7.1, 1.6 Hz, 2H), 7.77 (d, J = 9.3 Hz, 2H), 7.53 (t, J = 7.5 Hz, 2H), 5.83 (s, 1H), 4.00 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 154.0, 139.8, 137.5, 132.5, 129.0, 125.0, 124.4, 121.8, 116.8, 92.7, 38.2.
- 1-Propyl-4-((1-propylquinolin-4(1H)-ylidene)methyl)quinolin-1-ium iodide (14): using GP4, from 4-methyl-1-propylquinolin-1-ium 4-methylbenzenesulfonate (357 mg) and 1-propylquinolin-1-ium 4-methylbenzenesulfonate (343 mg). Anion exchange was made by reflux in a saturated potassium iodide solution (10 mL). Yield: 58%; dark green crystals; m.p. 229–231 °C; vis λmax (EtOH): 593 nm, log ε = 4.69; IR λmax (KBr): 3453, 3011, 2969, 2930, 2872, 1592, 1502, 1491, 1390, 1320, 1218, 1151, 1066, 790, 754, 642, 507 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.68 (dd, J = 8.7, 1.4 Hz, 2H), 8.22 (d, J = 7.3 Hz, 2H), 8.00 (dd, J = 8.8, 1.1 Hz, 2H), 7.89 (ddd, J = 8.6, 6.9, 1.3 Hz, 2H), 7.66 (d, J = 7.2 Hz, 2H), 7.63 (ddd, J = 7.6, 7.0, 1.1 Hz, 2H), 7.24 (s, 1H), 4.45 (t, J = 7.3 Hz, 4H), 1.84 (sx, J = 7.4 Hz, 4H), 0.95 (t, J = 7.4 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 148.9, 142.9, 137.6, 132.7, 126.1, 126.0, 125.3, 117.6, 108.6, 96.5, 54.8, 22.1, 10.7; ESI-HRMS calcd for [M-I]+ C25H27N2+ 355.2169, found 355.2162.
3.1.3. General Procedure for the Synthesis of Trimethine Cyanine Dyes 15 and 16
3.1.4. General Procedure for the Synthesis of Heptamethine Cyanine Dyes 17–19
- 2-(2-(2-Chloro-3-(2-(3-pentylbenzo[d]thiazol-2(3H)-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-3-pentylbenzo[d]thiazol-3-ium iodide (17): from 2-methyl-3-pentylbenzo[d]thiazol-3-ium iodide (2 × 347 mg). Yield: 56%; green crystals; m.p. 227–228 °C; vis λmax (EtOH): 801 nm, log ε = 5.45; IR λmax (KBr): 3058, 3000, 2925, 2859, 1579, 1530, 1503, 1459, 1428, 1403 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 7.95 (d, J = 7.9 Hz, 2H), 7.78 (d, J = 13.4 Hz, 2H), 7.72 (d, J = 8.3 Hz, 2H), 7.54 (t, J = 7.8 Hz, 2H), 7.36 (t, J = 7.7 Hz, 2H), 6.45 (d, J = 13.5 Hz, 2H), 4.39 (t, J = 6.8 Hz, 4H), 2.65 (t, J = 6.2 Hz, 4H), 1.83 (q, J = 5.5 Hz, 2H), 1.71 (p, J = 6.9 Hz, 4H), 1.46–1.26 (m, 8H), 0.87 (t, J = 6.6 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 163.4, 144.2, 141.6, 140.5, 128.2, 125.4, 125.2, 124.3, 123.1, 113.6, 100.0, 46.1, 28.1, 27.1, 26.5, 21.8, 20.4, 13.9.
- 2-(2-(2-Chloro-3-(2-(3-decylbenzo[d][1,3]selenazol-2(3H)-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-3-decylbenzo[d][1,3]selenazol-3-ium iodide (18): from 3-decyl-2-methylbenzoselenazol-3-ium iodide (2 × 464 mg). Yield: 44%; green crystals; m.p. 221–224 °C; vis λmax (EtOH): 814 nm, log ε = 5.46; IR λmax (KBr): 3060, 2921, 2850, 1660, 1578, 1524, 1503, 1450, 1428, 1399 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.04 (d, J = 7.9 Hz, 2H), 7.70 (d, J = 10.2 Hz, 4H), 7.59–7.49 (m, 2H), 7.35 (t, J = 7.6 Hz, 2H), 6.60 (d, J = 13.2 Hz, 2H), 4.42 (t, J = 6.9 Hz, 4H), 2.73–2.62 (m, 4H), 1.91–1.79 (m, 2H), 1.71 (p, J = 6.9 Hz, 4H), 1.45–1.16 (m, 28H), 0.84 (t, J = 6.9 Hz, 6H); 13C NMR (101 MHz, DMSO-d6): δ 168.5, 144.1, 143.1, 142.3, 128.2, 126.4, 125.4, 125.2, 125.1, 115.1, 103.8, 46.9, 31.3, 28.9, 28.7, 28.6, 27.2, 26.7, 25.8, 22.1, 14.0.
- 2-(2-(2-Chloro-3-(2-(3,3-dimethyl-1-(4-sulfobutyl)indolin-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-3,3-dimethyl-1-(4-sulfobutyl)-3H-indol-1-ium iodide (19-IR-783): from 2,3,3-trimethyl-1-(4-sulfobutyl)-3H-indol-1-ium iodide (2 × 423 mg). Yield: 65%; blue crystals; m.p. 220–225 °C; vis λmax (EtOH): 787 nm, log ε = 4.98; IR λmax (KBr): 3052, 2912, 2853, 1729, 1549, 1450, 1304, 1253, 1177, 1087, 975 cm−1; 1H NMR (400 MHz, DMSO-d6): δ 8.26 (d, J = 14.1 Hz, 2H), 7.99 (s, 2H), 7.62 (d, J = 7.4 Hz, 2H), 7.49 (d, J = 7.9 Hz, 2H), 7.42 (t, J = 7.7 Hz, 2H), 7.28 (t, J = 7.4 Hz, 2H), 6.37 (d, J = 14.1 Hz, 2H), 4.22 (t, J = 7.4 Hz, 4H), 2.73 (t, J = 6.1 Hz, 4H), 1.90–1.77 (m, 8H), 1.77–1.69 (m, 6H), 1.67 (s, 12H); 13C NMR (101 MHz, DMSO-d6): δ 172.1, 148.0, 143.1, 142.1, 141.1, 128.7, 126.3, 125.1, 122.5, 111.7, 101.8, 50.7, 49.0, 43.8, 36.5, 27.5, 26.1, 25.9, 22.5.
3.2. In Vitro Studies
3.2.1. Antiproliferative Effects in Human Cell Lines
Cell Culture
Cell Viability Using an MTT Assay
Photocytotoxicity Evaluation
Photostability Evaluation
Morphological Analysis
3.2.2. Flow Cytometry
3.3. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; De Hollanda, L.M.; Santini, A.; Reis, L.V.; Souto, S.B.; Souto, E.B.; Silva, A.M. Advances in nanobiomaterials for oncology nanomedicine. In Nanobiomaterials in Cancer Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 91–115. [Google Scholar] [CrossRef]
- Shindy, H.A. Characterization, mechanisms and applications in the chemistry of cyanine dyes: A review. Eur. J. Mol. Biotechnol. 2016, 14, 158–170. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, T.; Zhang, M.; Zhang, Q.; Wu, F.; Liu, Y.; Zhang, L.; Zhang, J.; Wang, M.; Sun, Y. Tumor-targeting multi-shelled hollow nanospheres as drug loading platforms for imaging-guided combinational cancer therapy. Biomater. Sci. 2020, 8, 1748–1758. [Google Scholar] [CrossRef]
- Conceição, S.D.; Ferreira, P.D.; Ferreira, F.V.L. Photochemistry and cytotoxicity evaluation of heptamethinecyanine near infrared (NIR) dyes. Int. J. Mol. Sci. 2013, 14, 18557–18571. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Dichiara, M.; Prezzavento, O.; Marrazzo, A.; Pittalà, V.; Salerno, L.; Rescifina, A.; Amata, E. Recent advances in drug discovery of phototherapeutic non-porphyrinic anticancer agents. Eur. J. Med. Chem. 2017, 142, 459–485. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Chen, W.; Tan, Y.; Chen, H.; Yin, J. Photodynamic therapy based on organic small molecular fluorescent dyes. Chin. Chem. Lett. 2019, 30, 1689–1703. [Google Scholar] [CrossRef]
- Williams, C.G. XXVI.—Researches on chinoline and its homologues. Trans. R. Soc. Edinb. 1857, 21, 377–401. [Google Scholar] [CrossRef] [Green Version]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dyes Pigments 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Ilina, K.; Henary, M. Cyanine dyes containing quinoline moieties: History, synthesis, optical properties, and applications. Chem. Eur. J. 2021, 27, 4230–4248. [Google Scholar] [CrossRef] [PubMed]
- Gopika, G.S.; Prasad, P.M.H.; Lekshmi, A.G.; Lekshmypriya, S.; Sreesaila, S.; Arunima, C.; Kumar, M.S.; Anil, A.; Sreekumar, A.; Pillai, Z.S. Chemistry of cyanine dyes-A review. Mater. Today Proc. 2021, 46, 3102–3108. [Google Scholar] [CrossRef]
- Yoshida, Z.; Kitao, T. Chemistry of Functional Dyes; Mita Press: Tokyo, Japan, 1989. [Google Scholar]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef]
- Bilici, K.; Cetin, S.; Aydındogan, E.; Yagci Acar, H.; Kolemen, S. Recent advances in cyanine-based phototherapy agents. Front. Chem. 2021, 9, 707876. [Google Scholar] [CrossRef] [PubMed]
- Dereje, D.M.; Pontremoli, C.; Moran Plata, M.J.; Visentin, S.; Barbero, N. Polymethine dyes for PDT: Recent advances and perspectives to drive future applications. Photochem. Photobiol. Sci. 2022, 21, 397–419. [Google Scholar] [CrossRef]
- Santos, P.F.; Reis, L.V.; Almeida, P.; Lynch, D.E. Crystal structures of a benzoselenazole-derived squarylium cyanine dye and three derivatives substituted at the central squaric ring. CrystEngComm 2011, 13, 1333–1338. [Google Scholar] [CrossRef]
- Boto, R.E.F.; El-Shishtawy, R.M.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and characterization of novel mono- and dicarboxyalkylthiacarbocyanines and their ester derivatives. Dyes Pigments 2007, 73, 195–205. [Google Scholar] [CrossRef]
- Lima, E.; Barroso, A.G.; Sousa, M.A.; Ferreira, O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Santos, A.O.; Reis, L.V. Picolylamine-functionalized benz[e]indole squaraine dyes: Synthetic approach, characterization and in vitro efficacy as potential anticancer phototherapeutic agents. Eur. J. Med. Chem. 2022, 229, 114071. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Gomes, V.S.D.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Synthesis and in vitro evaluation of the antitumoral phototherapeutic potential of squaraine cyanine dyes derived from indolenine. Dyes Pigments 2019, 167, 98–108. [Google Scholar] [CrossRef]
- Friães, S.; Silva, A.M.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Souto, E.B.; Almeida, P.; Ferreira, L.F.V.; Reis, L.V. Synthesis, spectroscopic characterization and biological evaluation of unsymmetrical aminosquarylium cyanine dyes. Bioorganic Med. Chem. 2017, 25, 3803–3814. [Google Scholar] [CrossRef]
- Nunes, S.C.; Ferreira, C.B.; Hümmer, J.; Ferreira, R.A.S.; Carlos, L.D.; Almeida, P.; de Zea Bermudez, V. Lamellar mono-amidosil hybrids incorporating monomethinecyanine dyes. J. Mater. Chem. C 2013, 1, 2290–2301. [Google Scholar] [CrossRef]
- Boto, R.E.F.; Anyanwu, U.; Sousa, F.; Almeida, P.; Queiroz, J.A. Thiacarbocyanine as ligand in dye-affinity chromatography for protein purification. II. Dynamic binding capacity using lysozyme as a model. Biomed. Chromatogr. 2009, 23, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.S.D.; Ferreira, J.C.C.; Boto, R.E.F.; Almeida, P.; Fernandes, J.R.; Sousa, M.J.; Gonçalves, M.S.T.; Reis, L.V. Squaraine dyes derived from indolenine and benzo[e]indole as potential fluorescent probes for HSA detection and antifungal agents. Photochem. Photobiol. 2022, 98. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Almeida, P. A new Vilsmeier-type reaction for one-pot synthesis of pH sensitive fluorescent cyanine dyes. Tetrahedron 2006, 62, 7793–7798. [Google Scholar] [CrossRef]
- Yoshino, Y.; Sato, Y.; Nishizawa, S. Deep-red light-up signaling of benzo[c,d]indole–quinoline monomethine cyanine for imaging of nucleolar RNA in living cells and for sequence-selective RNA analysis. Anal. Chem. 2019, 91, 14254–14260. [Google Scholar] [CrossRef] [PubMed]
- Kurutos, A.; Orehovec, I.; Tomašić Paić, A.; Crnolatac, I.; Horvat, L.; Gadjev, N.; Piantanida, I.; Deligeorgiev, T. New series of non-toxic DNA intercalators, mitochondria targeting fluorescent dyes. Dyes Pigments 2018, 148, 452–459. [Google Scholar] [CrossRef]
- Crnolatac, I.; Tumir, L.-M.; Lesev, N.Y.; Vasilev, A.A.; Deligeorgiev, T.G.; Mišković, K.; Glavaš-Obrovac, L.; Vugrek, O.; Piantanida, I. Probing the structural properties of DNA/RNA grooves with sterically restricted phosphonium dyes: Screening of dye cytotoxicity and uptake. ChemMedChem 2013, 8, 1093–1103. [Google Scholar] [CrossRef]
- Glavaš-Obrovac, L.; Piantanida, I.; Marczi, S.; Mašić, L.; Timcheva, I.I.; Deligeorgiev, T.G. Minor structural differences of monomethine cyanine derivatives yield strong variation in their interactions with DNA, RNA as well as on their in vitro antiproliferative activity. Bioorganic Med. Chem. 2009, 17, 4747–4755. [Google Scholar] [CrossRef]
- Kaloyanova, S.; Crnolatac, I.; Lesev, N.; Piantanida, I.; Deligeorgiev, T. Synthesis and study of nucleic acids interactions of novel monomethine cyanine dyes. Dyes Pigments 2012, 92, 1184–1191. [Google Scholar] [CrossRef]
- Mills, W.H. LIV.—The cyanine dyes. Part IV. Cyanine dyes of the benzothiazole series. J. Chem. Soc. Trans. 1922, 121, 455–466. [Google Scholar] [CrossRef]
- Kendall, J.D.; Suggate, H.G. 319. The reactivity of the alkylthio-group in nitrogen ring compounds. Part I. A general method for the preparation of symmetrical and unsymmetrical thiacyanines. J. Chem. Soc. 1949, 1949, 1503–1509. [Google Scholar] [CrossRef]
- Hamer, F.M. XXXI.—The ψ-cyanine condensation. J. Chem. Soc. 1928, 1928, 206–215. [Google Scholar] [CrossRef]
- Fisher, N.I.; Hamer, F.M. 200. Oxacyanines. J. Chem. Soc. 1934, 1934, 962–965. [Google Scholar] [CrossRef]
- Boto, R.E.F.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and characterization of mono- and dicarboxyalkyloxacarbocyanines. Dyes Pigments 2007, 75, 298–305. [Google Scholar] [CrossRef]
- Boto, R.E.F.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and characterization of new mono- and dicarboxyalkylselenacarbocyanines. Dyes Pigments 2008, 76, 165–172. [Google Scholar] [CrossRef]
- Pais, I.R.; Nunes, M.J.; Reis, L.V.; Santos, P.F.; Almeida, P. The synthesis of chloroheptamethinecyanine dyes in the absence of water. Dyes Pigments 2008, 77, 48–52. [Google Scholar] [CrossRef]
- Hamer, F.M. 149. Bases of which methincyanines are the quaternary salts. J. Chem. Soc. 1940, 1940, 799–808. [Google Scholar] [CrossRef]
- Knott, E.B. Compounds containing sulphur chromophores. Part V. Complex cyanines. J. Chem. Soc. 1955, 1955, 949–954. [Google Scholar] [CrossRef]
- Hamer, F.M. CCCLXXIV.—A general method for the preparation of carbocyanine dyes. J. Chem. Soc. 1927, 1927, 2796–2804. [Google Scholar] [CrossRef]
- Clark, L.M. CCCIII.—The methylene bases from 1-methylbenzthiazole and 1-methylbenzselenazole methiodides; with a note on the preparation of 1-substituted benzthiazoles. J. Chem. Soc. 1928, 1928, 2313–2320. [Google Scholar] [CrossRef]
- Narayanan, N.; Patonay, G. A new method for the synthesis of heptamethine cyanine dyes: Synthesis of new near-infrared fluorescent labels. J. Org. Chem. 1995, 60, 2391–2395. [Google Scholar] [CrossRef]
- Henary, M.; Paranjpe, S.; Owens, E.A. Synthesis and applications of benzothiazole containing cyanine dyes. Heterocycl. Commun. 2013, 19, 1–11. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Morrow, J.P.; Pizzi, D.; Azizah, A.M.; Davis, T.P.; Tabor, R.F.; Kempe, K. Tuning cellular interactions of carboxylic acid-side-chain-containing polyacrylates: The role of cyanine dye label and side-chain type. Biomacromolecules 2020, 21, 3007–3016. [Google Scholar] [CrossRef]
- Nödling, A.R.; Mills, E.M.; Li, X.; Cardella, D.; Sayers, E.J.; Wu, S.-H.; Jones, A.T.; Luk, L.Y.P.; Tsai, Y.-H. Cyanine dye mediated mitochondrial targeting enhances the anti-cancer activity of small-molecule cargoes. Chem. Commun. 2020, 56, 4672–4675. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine conjugates in cancer theranostics. Bioact. Mater. 2021, 6, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.J.; Park, T.I.H.; Cooper, E.; Dragunow, M.; Denny, W.A.; Jose, J. Heptamethine cyanine dye mediated drug delivery: Hype or hope. Bioconjugate Chem. 2020, 31, 1724–1739. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, D.; Bates, M.; Jayaram, M.; Young, M.; He, J.; Raithel, A.L.; Hamann, T.W.; Zhang, W.; Borhan, B.; Lunt, R.R.; et al. Modulating cellular cytotoxicity and phototoxicity of fluorescent organic salts through counterion pairing. Sci. Rep. 2019, 9, 15288. [Google Scholar] [CrossRef]
- Yang, Y.; Komaki, Y.; Kimura, S.Y.; Hu, H.-Y.; Wagner, E.D.; Mariñas, B.J.; Plewa, M.J. Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ. Sci. Technol. 2014, 48, 12362–12369. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Matzke, M.; Stock, F.; Thiele, K.; Uerdingen, M.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8, 621–629. [Google Scholar] [CrossRef]
- Yan, G.; Elbadawi, M.; Efferth, T. Multiple cell death modalities and their key features (Review). World Acad. Sci. J. 2020, 2, 39–48. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Wang, Y.K.; Jiang, K.; Gai, X.D. Sorbitol induces apoptosis of human colorectal cancer cells via p38 MAPK signal transduction. Oncol. Lett. 2014, 7, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shen, L.; Yu, M.; Ni, J.; Dong, X.; Zhou, Y.; Wu, S. Colon cancer cells treated with 5-fluorouracil exhibit changes in polylactosamine-type N-glycans. Mol. Med. Rep. 2014, 9, 1697–1702. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Bruns, R.F.; Watson, I.A. Rules for identifying potentially reactive or promiscuous compounds. J. Med. Chem. 2012, 55, 9763–9772. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.A.; Drexhage, K.H. Stable heptamethine pyrylium dyes that absorb in the infrared. J. Org. Chem. 1977, 42, 885–888. [Google Scholar] [CrossRef]
- Tipson, R.S. On esters of p-toluenesulfonic acid. J. Org. Chem. 1944, 09, 235–241. [Google Scholar] [CrossRef]
- Özkınalı, S. Spectroscopic and thermal properties of newly mixed azocalix[4]arene ester derivatives. Dyes Pigments 2014, 107, 81–89. [Google Scholar] [CrossRef]
- Pardal, A.C.; Ramos, S.S.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and spectroscopic characterisation of N-alkyl quaternary ammonium salts typical precursors of cyanines. Molecules 2002, 7, 320–330. [Google Scholar] [CrossRef]
- Catarro, M.; Serrano, J.; Cavalheiro, E.; Ramos, S.; Santos, A.O.; Silvestre, S.; Almeida, P. Novel 4-acetamide-2-alkylthio-N-acetanilides resembling nimesulide: Synthesis, cell viability evaluation and in silico studies. Bioorganic Med. Chem. 2017, 25, 4304–4313. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. (Eds.) Purification of Organic Chemicals. In Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2009; Chapter 4; pp. 88–444. [Google Scholar] [CrossRef]
- Ramos, S.S.; Santos, P.F.; Reis, L.V.; Almeida, P. Some new symmetric rigidified triheterocyclic heptamethinecyanine dyes absorbing in the near infrared. Dyes Pigments 2002, 53, 143–152. [Google Scholar] [CrossRef]










 | |||||||
|---|---|---|---|---|---|---|---|
| Dye | R | X | W | Yield (%) | m.p. (°C) | λmax (nm) | Log ε |
| 2 a | CH3 | S | Cl | 68 | 265–266 (lit. 269 [32]) | 424 | 4.97 |
| 3 a | CH3 | S | Br | 69 | 302–303 (lit. 292–293 [40]) | 424 | 4.83 |
| 4 a | CH3 | S | I | 94 | 315–316 (lit. 303 [33]) | 424 | 4.96 |
| 5 a | CH3 | S | TsO | 77 | 296–297 | 424 | 4.98 |
| 6 a | C2H5 | S | TsO | 80 | 194–196 | 425 | 4.84 |
| 7 a | C2H5 | S | ClO4 | 83 | 296–297 | 425 | 4.92 |
| 8 b | CH2C6H5 | S | I | 25 | 249–250 | 427 | 4.87 |
| 9 b | CH2CO2CH3 | S | Br | 28 | 226–227 | 427 | 5.13 |
| 10 b | C5H11 | S | I | 75 | 164–166 | 426 | 4.77 |
| 11 b | C10H21 | S | I | 48 | 232–233 | 427 | 4.92 |
| 12 a | CH3 | O | TsO | 66 | 297–298 | 376 | 4.96 |
| 13 c | CH3 | CH=CH | I | 76 | 240–241 (lit. 245–246 [34]) | 523 | 4.60 |
| 14 d |  | TsO | 58 | 229–231 | 593 | 4.69 | |
| 15 e | C2H5 | O | I | 24 | 281–282 (lit. 277–279 [41]) | 485 | 5.15 |
| 16 e | C2H5 | Se | I | 95 | 272–273 (lit. 270–271 [42]) | 573 | 4.94 |
| 17 f | C5H11 | S | I | 56 | 227–228 | 801 | 5.45 |
| 18 f | C10H21 | Se | I | 44 | 221–224 | 814 | 5.46 |
| 19 f (IR-783) | C4H9O3S | C(CH3)2 | I | 65 | 223–225 | 787 | 4.98 |
| Dye | NHDF | Caco-2 | MCF-7 | PC-3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | 95% CI | IC50 | 95% CI | SI | IC50 | 95% CI | SI | IC50 | 95% CI | SI | |
| 5-FU | 6.17 | 3.05–12.48 | 1.70 | 0.88–3.17 | 3.6 | 1.24 | 0.89–1.73 | 1.7 | 1.72 | 0.64–4.65 | 3.6 |
| 2 | 0.77 | 0.65–0.90 | 0.65 | 0.48–0.88 | 1.2 | - | - | - | - | - | - |
| 3 | 0.41 | 0.33–0.50 | 0.52 | 0.38–0.71 | 0.8 | - | - | - | - | - | - |
| 4 | 0.73 | 0.48–1.00 | 0.40 | 0.26–0.59 | 1.8 | - | - | - | - | - | - |
| 5 | 0.68 | 0.52–0.87 | 0.28 | 0.20–0.41 | 2.4 | - | - | - | - | - | - |
| 6 | 0.49 | 0.39–0.62 | 0.30 | 0.23–0.39 | 1.6 | - | - | - | - | - | - |
| 7 | 0.83 | 0.58–1.16 | 0.28 | 0.13–0.64 | 3.0 | - | - | - | - | - | - |
| 8 | 0.93 | 0.73–1.18 | 0.95 | 0.68–1.32 | 1.0 | - | - | - | - | - | - |
| 10 | 0.67 | 0.57–0.78 | 1.00 | 0.63–1.55 | 0.7 | - | - | - | - | - | - |
| 11 | 0.72 | 0.56–0.94 | 1.24 | 0.88–1.75 | 0.6 | - | - | - | - | - | - |
| 12 | 13.95 | 7.63–25.51 | 0.67 | 0.39–1.15 | 20.9 | 4.57 | 1.73–12.07 | 4.5 | 4.53 | 1.99–10.29 | 3.1 |
| 13 | 0.59 | 0.46–0.75 | 0.58 | 0.39–0.85 | 1.0 | - | - | - | - | - | - |
| 14 | 0.99 | 0.80–1.26 | 0.68 | 0.44–1.01 | 1.5 | - | - | - | - | - | - |
| 15 | 4.02 | 1.54–10.25 | 0.39 | 0.29–0.51 | 10.4 | - | - | - | - | - | - |
| 16 | 0.48 | 0.34–0.67 | 0.07 | 0.05–0.10 | 6.5 | - | - | - | - | - | - |
| 17 | 0.91 | 0.69–1.20 | 2.06 | 1.41–2.97 | 0.4 | - | - | - | - | - | - |
| Descriptor | Value | |
|---|---|---|
| Physicochemical Properties | H-bond donors | 0 |
| H-bond acceptors | 2 | |
| Molecular weight (g/mol) | 279.31 | |
| Consensus CLogP | 2.27 | |
| Molar refractivity | 82.87 | |
| Rotatable bonds | 1 | |
| Fraction Csp3 | 0.12 | |
| TPSA (Ų) | 35.09 | |
| Log S (ESOL) | −4.57 (moderately soluble) | |
| Druglikeness | Lipinski | Yes, 0 violations |
| Ghose | Yes | |
| Veber | Yes | |
| Egan | Yes | |
| Muegge | Yes | |
| Bioavailability score | 0.55 | |
| Medicinal Chemistry | PAINS | 0 alert |
| Brenk | 1 alert: quaternary nitrogen | |
| Leadlikeness | Yes | |
| Synthetic accessibility | 3.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.L.; Maia, A.; Santos, A.O.; Lima, E.; Reis, L.V.; Nunes, M.J.; Boto, R.E.F.; Silvestre, S.; Almeida, P. An Insight into Symmetrical Cyanine Dyes as Promising Selective Antiproliferative Agents in Caco-2 Colorectal Cancer Cells. Molecules 2022, 27, 5779. https://doi.org/10.3390/molecules27185779
Serrano JL, Maia A, Santos AO, Lima E, Reis LV, Nunes MJ, Boto REF, Silvestre S, Almeida P. An Insight into Symmetrical Cyanine Dyes as Promising Selective Antiproliferative Agents in Caco-2 Colorectal Cancer Cells. Molecules. 2022; 27(18):5779. https://doi.org/10.3390/molecules27185779
Chicago/Turabian StyleSerrano, João L., Ana Maia, Adriana O. Santos, Eurico Lima, Lucinda V. Reis, Maria J. Nunes, Renato E. F. Boto, Samuel Silvestre, and Paulo Almeida. 2022. "An Insight into Symmetrical Cyanine Dyes as Promising Selective Antiproliferative Agents in Caco-2 Colorectal Cancer Cells" Molecules 27, no. 18: 5779. https://doi.org/10.3390/molecules27185779