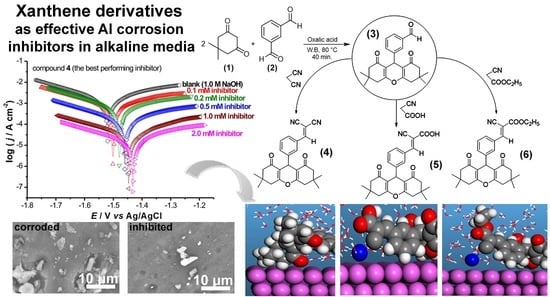
Synthesis of Cyano-Benzylidene Xanthene Synthons Using a Diprotic Brønsted Acid Catalyst, and Their Application as Efficient Inhibitors of Aluminum Corrosion in Alkaline Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Electrochemical Measurements
2.4. Computational Details
2.4.1. Quantum Chemical Calculations on Isolated Molecules
2.4.2. Monte Carlo Simulations
3. Results and Discussion
3.1. Chemistry
3.2. Polarization Studies
3.3. Characterization of the Corroded and Inhibited Al Surfaces
3.4. Computational Study
3.4.1. DFT Modelling
3.4.2. Monte Carlo Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jacques, M. Corrosion of Aluminum; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Li, X.; Nie, X.; Wang, L.; Northwood, D.O. Corrosion protection properties of anodic oxide coatings on an Al–Si alloy. Surf. Coat. Technol. 2005, 200, 1994. [Google Scholar] [CrossRef]
- Talbot, D.; Talbot, J. Corrosion Science and Technology; CRC Press LLC: Boca Raton, FL, USA, 1998. [Google Scholar]
- Hurlen, T.; Lian, H.; Odegard, O.; Valand, T. Corrosion and passive behaviour of aluminum in weakly acid solution. Electrochim. Acta 1984, 29, 579. [Google Scholar] [CrossRef]
- Zhang, J.; Klasky, M.; Letellier, B.C. The aluminum chemistry and corrosion in alkaline solutions. J. Nucl. Mater. 2009, 384, 175. [Google Scholar] [CrossRef]
- Prabhu, D.; Rao, P. Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab. J. Chem. 2017, 10, S2234. [Google Scholar]
- Boukerche, I.; Djerad, S.; Benmansour, L.; Tifouti, L.; Saleh, K. Degradability of aluminum in acidic and alkaline solutions. Corros. Sci. 2014, 78, 343. [Google Scholar] [CrossRef]
- Mori, R. Recent Developments for Aluminum–Air Batteries. Electrochem. Energy Rev. 2020, 3, 344. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246. [Google Scholar] [CrossRef]
- Kendig, M.W.; Buchheit, R.G. Corrosion Inhibition of Aluminum and Aluminum Alloys by Soluble Chromates, Chromate Coatings, and Chromate-Free Coatings. Corrosion 2003, 59, 379. [Google Scholar] [CrossRef]
- Xhanari, K.; Finšgar, M. Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab. J. Chem. 2019, 12, 4646. [Google Scholar] [CrossRef]
- Sastri, V.S. Corrosion Inhibitors: Principles and Applications; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Xhanari, K.; Finšgar, M.; Hrnčič, M.K.; Maver, U.; Knez, Ž.; Seiti, B. Green corrosion inhibitors for aluminium and its alloys: A review. RSC Adv. 2017, 7, 27299. [Google Scholar] [CrossRef]
- Sanyal, B. Organic compounds as corrosion inhibitors in different environments—A review. Prog. Org. Coat. 1981, 9, 165. [Google Scholar] [CrossRef]
- Evans, U.R. The Corrosion and Oxidation of Metals: 1st Supplementary Volume; St. Martin’s Press: New York, NY, USA, 1968. [Google Scholar]
- Ghulam, S.; Aamer, S.; Ali, C.P. A Review on the Recent Trends in Synthetic Strategies and Applications of Xanthene Dyes. Mini Rev. Org. Chem. 2018, 15, 166. [Google Scholar]
- Lambert, R.; Martin, J.; Merrett, J.; Parkes, K.; Thomas, G. PCT Int. Appl. WO 9706178. Chem. Abstr. 1997, 126, 212377y. [Google Scholar]
- Peres, V.; Nagem, T.J.; de Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683. [Google Scholar] [CrossRef]
- Ahmad, M.; King, T.A.; Ko, D.-K.; Cha, B.H.; Lee, J. Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases. J. Phys. D Appl. Phys 2002, 35, 1473. [Google Scholar] [CrossRef]
- Knight, C.G.; Stephens, T. Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. Biochem. J. 1989, 258, 683. [Google Scholar] [CrossRef]
- Ignatushchenko, M.V.; Winter, R.W.; Riscoe, M. Xanthones as antimalarial agents: Stage specificity. Am. J. Trop. Med. 2000, 62, 77. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N.; Eseola, A. Anticorrosion potential of 2-mesityl-1H-imidazo [4, 5-f][1, 10] phenanthroline on mild steel in sulfuric acid solution: Experimental and theoretical study. Ind. Eng. Chem. Res. 2011, 50, 2098. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.; Essien, K.; Obot, I. Computational simulation and corrosion inhibitive potential of alloxazine for mild steel in 1 M HCl. J. Comp. Methods Mol. Des. 2011, 1, 26. [Google Scholar]
- Cariou, C.C.; Clarkson, G.J.; Shipman, M. Rapid synthesis of 1, 3, 4, 4-tetrasubstituted β-lactams from methyleneaziridines using a four-component reaction. J. Org. Chem. 2008, 73, 9762. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B. 01; Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378. [Google Scholar] [CrossRef] [PubMed]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E.J. Equations of State Calculations by Fast Computing Machines. J. Chem. Phys. 1953, 21, 1087. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Materials Studio, 17.1.0.48; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
- Xu, L.; Lin, J.; Bai, Y.; Mavrikakis, M. Atomic and molecular adsorption on Cu (111). Top. Catal. 2018, 61, 736. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. A guide to polarisation curve interpretation: Deconstruction of experimental curves typical of the Fe/H2O/H+/O2 corrosion system. Corros. Sci. 2005, 47, 2125. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarisation curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves. Corros. Sci. 2005, 47, 3178. [Google Scholar] [CrossRef]
- Rosborg, B.; Pan, J.; Leygraf, C. Tafel slopes used in monitoring of copper corrosion in a bentonite/groundwater environment. Corros. Sci. 2005, 47, 3267. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2004.
- Metikos-Hukovic, M.; Grubac, Z.; Stupnisek-Lisac, E. Organic corrosion inhibitors for aluminum in perchloric acid. Corrosion 1994, 50, 146. [Google Scholar] [CrossRef]
- Li, W.; Cochell, T.; Manthiram, A. Activation of aluminum as an effective reducing agent by pitting corrosion for wet-chemical analysis. Sci. Rep. 2013, 3, 1. [Google Scholar]
- Vigh, A.K. Oxide Films: Influence of solid-state properties on electrochemical behavior. In Oxide and Oxide Films; Diggle, J.W., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1972; Volume 2. [Google Scholar]
- Kobotiatis, L.; Pebere, N.; Koutsoukos, P.G. Study of the electrochemical behaviour of the 7075 aluminum alloy in the presence of sodium oxalate. Corros. Sci. 1999, 41, 941. [Google Scholar] [CrossRef]
- Ryl, J.; Wysocka, J.; Cieslik, M.; Gerengi, H.; Ossowski, T.; Krakowiak, S.; Niedzialkowski, P. Understanding the origin of high corrosion inhibition efficiency of bee products towards aluminium alloys in alkaline environments. Electrochim. Acta 2019, 304, 263. [Google Scholar] [CrossRef]
- Ryl, J.; Wysocka, J.; Jarzynka, M.; Zielinski, A.; Orlikowski, J.; Darowicki, K. Effect of native air-formed oxidation on the corrosion behavior of AA 7075 aluminum alloys. Corros. Sci. 2014, 87, 150. [Google Scholar] [CrossRef]
- Wysocka, J.; Krakowiak, S.; Ryl, J.; Darowicki, K. Investigation of the electrochemical behaviour of AA1050 aluminium alloy in aqueous alkaline solutions using Dynamic Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2016, 778, 126. [Google Scholar] [CrossRef]
- Lopez, D.A.; Schreiner, W.H.; de Sancher, S.R.; Simison, S.N. The influence of inhibitors molecular structure and steel microstructure on corrosion layers in CO2 corrosion: An XPS and SEM characterization. Appl. Surf. Sci. 2004, 236, 77. [Google Scholar] [CrossRef]
- Sobaszek, M.; Siuzdak, K.; Ryl, J.; Sawczak, M.; Gupta, S.; Carrizosa, S.B.; Ficek, M.; Dec, B.; Darowicki, K.; Bogdanowicz, R. Diamond Phase (sp3-C) rich boron-doped carbon nanowalls (sp2-C): Physicochemical and electrochemical properties. J. Phys. Chem. C 2017, 121, 20821. [Google Scholar] [CrossRef]
- Mazzotta, E.; Rella, S.; Turco, A.; Malitesta, C. XPS in development of chemical sensors. RSC Adv. 2015, 5, 83164. [Google Scholar] [CrossRef]
- Sobaszek, M.; Siuzdak, K.; Ryl, J.; Bogdanowicz, R.; Swain, G.M. The electrochemical determination of isatin at nanocrystalline boron-doped diamond electrodes: Stress monitoring of animals. Sens. Actuators B Chem. 2020, 306, 127592. [Google Scholar] [CrossRef]
- Rodrigez-Valdez, L.M.; Martinez-Villafane, A.; Glossman-Mitnik, D.J. Computational simulation of the molecular struc-ture and properties of heterocyclic organic compounds with possible corrosion inhibition properties. Mol. Struct. 2005, 713, 65. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 13282. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29, 845. [Google Scholar] [CrossRef] [PubMed]










| Al (at.%) | Fe (at.%) | O (at.%) | C (at.%) | |
|---|---|---|---|---|
| Polished | 96.0 | 0.9 | 3.1 | 0.0 |
| Corroded | 78.8 | 0.9 | 18.3 | 2.0 |
| (3) | 78.6 | 1.2 | 12.5 | 7.8 |
| (4) | 86.7 | 1.0 | 9.0 | 3.4 |
| (5) | 85.5 | 1.1 | 10.3 | 3.1 |
| (6) | 91.5 | 1.0 | 5.3 | 2.1 |
| C 1s | Al 2p3/2 | O 1s | N 1s | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C-C /C=C | C-O | C-N /C=O | Al2O3 /AlOOH | Al2O3 * | Al2O3 /C=O | AlOOH/CO | H2Oads | C-N | |
| BE/eV | 285.4 | 286.8 | 288–291 | 75.4 | 77.2 | 531.4 | 532.8 | 534.4 | 400.5 |
| Corroded | 24.0 | 7.3 | 4.0 | 21.9 | - | 13.9 | 24.8 | 3.1 | - |
| (4) | 14.2 | 14.5 | 7.8 | 8.5 | 20.5 | 14.1 | 23.2 | 4.5 | 2.2 |
| Molecule | EHOMO, eV | ELUMO, eV | ∆E, eV | DM, D |
|---|---|---|---|---|
| (3) | −6.71 | −2.13 | 4.58 | 11.12 |
| (4) | −6.74 | −2.97 | 3.78 | 13.55 |
| (5) | −6.74 | −2.89 | 3.85 | 10.09 |
| (6) | −6.70 | −2.74 | 3.96 | 12.55 |
| Inhibitor | Total Energy (kcal/mol) | Adsorption Energy (kcal/mol) | Rigid Adsorption Energy (kcal/mol) | Deformation Energy (kcal/mol) | dEad/dNi | ||
|---|---|---|---|---|---|---|---|
| Inhibitor | NaOH | H2O | |||||
| (3) | −597 | −690 | −680 | −10 | −78 | −35 | −11 |
| (4) | −645 | −739 | −734 | −5 | −98 | −32 | −13 |
| (5) | −623 | −720 | −713 | −4 | −95 | −39 | −13 |
| (6) | −612 | −705 | −697 | −8 | −89 | −33 | −12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.A.; Mersal, G.A.M.; El-Hendawy, M.M.; Shaltout, A.A.; Badawi, A.; Boman, J.; Gobouri, A.A.; Saracoglu, M.; Kandemirli, F.; Boukherroub, R.; et al. Synthesis of Cyano-Benzylidene Xanthene Synthons Using a Diprotic Brønsted Acid Catalyst, and Their Application as Efficient Inhibitors of Aluminum Corrosion in Alkaline Solutions. Molecules 2022, 27, 5733. https://doi.org/10.3390/molecules27175733
Amin MA, Mersal GAM, El-Hendawy MM, Shaltout AA, Badawi A, Boman J, Gobouri AA, Saracoglu M, Kandemirli F, Boukherroub R, et al. Synthesis of Cyano-Benzylidene Xanthene Synthons Using a Diprotic Brønsted Acid Catalyst, and Their Application as Efficient Inhibitors of Aluminum Corrosion in Alkaline Solutions. Molecules. 2022; 27(17):5733. https://doi.org/10.3390/molecules27175733
Chicago/Turabian StyleAmin, Mohammed A., Gaber A. M. Mersal, Morad M. El-Hendawy, Abdallah A. Shaltout, Ali Badawi, Johan Boman, Adil A. Gobouri, Murat Saracoglu, Fatma Kandemirli, Rabah Boukherroub, and et al. 2022. "Synthesis of Cyano-Benzylidene Xanthene Synthons Using a Diprotic Brønsted Acid Catalyst, and Their Application as Efficient Inhibitors of Aluminum Corrosion in Alkaline Solutions" Molecules 27, no. 17: 5733. https://doi.org/10.3390/molecules27175733