Effect of Iron Complex Source on MWWTP Effluent Treatment by Solar Photo-Fenton: Micropollutant Degradation, Toxicity Removal and Operating Costs
Abstract
:1. Introduction
2. Results and Discussion
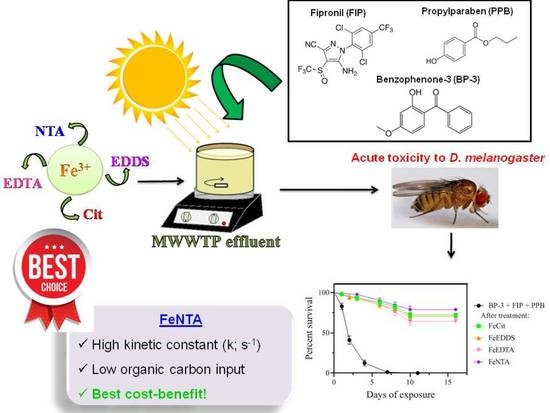
2.1. Influence of the Iron Complex Source, Fe/L Molar Ratios, and H2O2 Concentration
2.2. Acute Toxicity Assays: Drosophila Melanogaster Lifespan
2.3. Cost Assessment
3. Materials and Methods
3.1. Reagents
3.2. MWWTP Effluent
3.3. Photodegradation Experiments
3.4. Chemical and Bioassay Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.Y.; Ang, P.O.; Murphy, M.B.; Lam, P.K.S. Occurrence, Distribution, and Fate of Organic UV Filters in Coral Communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Rzemieniec, J.; Litwa, E.; Lasoń, W.; Kajta, M. Prenatal exposure to benzophenone-3 (BP-3) induces apoptosis, disrupts estrogen receptor expression and alters the epigenetic status of mouse neurons. J. Steroid Biochem. Mol. Biol. 2018, 182, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Chen, A.; Bian, Z.; Yang, Y.; Wang, H. In situ synthesis of a cadmium sulfide/reduced graphene oxide/bismuth Z-scheme oxyiodide system for enhanced photocatalytic performance in chlorinated paraben degradation. Chem. Eng. J. 2019, 359, 530–541. [Google Scholar] [CrossRef]
- Bielská, L.; Hale, S.E.; Škulcová, L. A review on the stereospecific fate and effects of chiral conazole fungicides. Sci. Total Environ. 2021, 750, 141600. [Google Scholar] [CrossRef]
- Kung, T.A.; Lee, S.H.; Yang, T.C.; Wang, W.H. Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan. Sci. Total Environ. 2018, 635, 1302–1307. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Ricardo, I.A.; Leitão, J.M.M.; Esteves da Silva, J.C.G. A review on advanced oxidation processes: From classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples. Trends Environ. Anal. Chem. 2019, 24, e00072. [Google Scholar] [CrossRef]
- Amildon Ricardo, I.; Paniagua, C.E.S.; Paiva, V.A.B.; Gonçalves, B.R.; Sousa, R.M.F.; Machado, A.E.H.; Trovó, A.G. Degradation and initial mechanism pathway of chloramphenicol by photo-Fenton process at circumneutral pH. Chem. Eng. J. 2018, 339, 531–538. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2021, 40, 16–19. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Somma, I.D.; Marotta, R.; Andreozzi, R. Applied Catalysis B: Environmental Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Ahile, U.J.; Wuana, R.A.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total Environ. 2020, 710, 134872. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Dantas, R.F.; Esplugas, S. Assessment of iron chelates efficiency forphoto-Fenton at neutral pH. Water Res. 2014, 61, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.D.; Marson, E.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.M.; Trovó, A.G. Contrasting the performance of photo-Fenton at neutral pH in the presence of different organic iron-complexes using hydrogen peroxide or persulfate as oxidants for naproxen degradation and removal of antimicrobial activity. Process Saf. Environ. Prot. 2021, 147, 798–807. [Google Scholar] [CrossRef]
- Doumic, L.I.; Soares, P.A.; Ayude, M.A.; Cassanello, M.; Boaventura, R.A.R.; Vilar, V.J.P. Enhancement of a solar photo-Fenton reaction by using ferrioxalate complexes for the treatment of a synthetic cotton-textile dyeing wastewater. Chem. Eng. J. 2015, 277, 86–96. [Google Scholar] [CrossRef]
- Sadaria, A.M.; Labban, C.W.; Steele, J.C.; Maurer, M.M.; Halden, R.U. Retrospective nationwide occurrence of fi pronil and its degradates in U.S. wastewater and sewage sludge from 2001–2016. Water Res. 2019, 155, 465–473. [Google Scholar] [CrossRef]
- Fang, W.; Peng, Y.; Muir, D.; Lin, J.; Zhang, X. A critical review of synthetic chemicals in surface waters of the US, the EU and China. Environ. Int. 2019, 131, 104994. [Google Scholar] [CrossRef]
- Paniagua, C.E.S.; Marson, E.O.; Ricardo, I.A.; Paiva, V.A.B.; Gonçalves, B.R.; Trovó, A.G. Matrix Effects on the Degradation of Gemfibrozil, Hydrochlorothiazide, and Naproxen by Heterogeneous Photocatalysis. J. Braz. Chem. Soc. 2020, 31, 1161–1169. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; Obra, I.D.L.; Miralles-Cuevas, S.; Gualda-Alonso, E.; López, J.L.C.; Pérez, J.A.S. Assessment of different iron sources for continuous flow solar photo-Fenton at neutral pH for sulfamethoxazole removal in actual MWWTP effluents. J. Water Process Eng. 2021, 42, 102109. [Google Scholar] [CrossRef]
- Pérez, J.A.S.; Arzate, S.; Soriano-molina, P.; Sánchez, J.L.G. Neutral or acidic pH for the removal of contaminants of emerging concern in wastewater by solar photo-Fenton ? A techno-economic assessment of continuous raceway pond reactors. Sci. Total Environ. 2020, 736, 139681. [Google Scholar] [CrossRef]
- Basin, V.; Kimosop, S.J.; Getenga, Z.M.; Orata, F. Residue levels and discharge loads of antibiotics in wastewater treatment plants ( WWTPs ), hospital lagoons, and rivers. Environ. Monit. Assess. 2016, 188, 532. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Bauerfeldt, G.F.; Castro, R.N.; Magalhães, V.S.; Alves, M.C.C. Determination of Fipronil and Fipronil—Sulfone in Surface Waters of the Guandu River Basin by High—Performance Liquid Chromatography with Mass Spectrometry. Bull. Environ. Contam. Toxicol. 2022, 108, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence, fate and risk assessment of parabens and their chlorinated derivatives in an advanced wastewater treatment plant. J. Hazard. Mater. 2015, 300, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Canosa, P.; Rodríguez, I.; Rubí, E.; Bollaín, M.H.; Cela, R. Optimisation of a solid-phase microextraction method for the determination of parabens in water samples at the low ng per litre level. J. Chromatogr. A 2006, 1124, 3–10. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, K. Mass loading and emission of benzophenone-3 (BP-3) and its derivatives in wastewater treatment plants in New York State, USA. Sci. Total Environ. 2017, 579, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Arfaeinia, H.; Asadgol, Z.; Ramavandi, B.; Dobaradaran, S.; Kalantari, R.R.; Poureshgh, Y.; Behroozi, M.; Asgari, E.; Asl, F.B.; Sahebi, S. Monitoring and eco-toxicity effect of paraben-based pollutants in sediments/seawater, north of the Persian Gulf. Environ. Geochem. Health 2022, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Juksu, K.; Zhao, J.L.; Liu, Y.S.; Yao, L.; Sarin, C.; Sreesai, S.; Klomjek, P.; Jiang, Y.X.; Ying, G.G. Occurrence, fate and risk assessment of biocides in wastewater treatment plants and aquatic environments in Thailand. Sci. Total Environ. 2019, 690, 1110–1119. [Google Scholar] [CrossRef]
- Derisso, C.R.; Pompei, C.M.E.; Spadoto, M.; da Silva Pinto, T.; Vieira, E.M. Occurrence of Parabens in Surface Water, Wastewater Treatment Plant in Southeast of Brazil and Assessment of Their Environmental Risk. Water. Air. Soil Pollut. 2020, 231, 468. [Google Scholar] [CrossRef]
- Hopkins, Z.R.; Blaney, L. An aggregate analysis of personal care products in the environment: Identifying the distribution of environmentally-relevant concentrations. Environ. Int. 2016, 92–93, 301–316. [Google Scholar] [CrossRef]
- Semones, M.C.; Sharpless, C.M.; MacKay, A.A.; Chin, Y.P. Photodegradation of UV filters oxybenzone and sulisobenzone in wastewater effluent and by dissolved organic matter. Appl. Geochem. 2017, 83, 150–157. [Google Scholar] [CrossRef]
- Montagner, C.C.; Vidal, C.; Acayaba, R.D. Emerging contaminants in aquatic matrices from Brazil: Current scenario and analytical, ecotoxicological and legislational aspects. Quim. Nova 2017, 40, 1094–1110. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; da Silva, V.M.; de Oliveira Silva, J.; da Hora Machado, A.E.; Trovó, A.G. Coupling coagulation, flocculation and decantation with photo-Fenton process for treatment of industrial wastewater containing fipronil: Biodegradability and toxicity assessment. J. Environ. Manag. 2016, 174, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, I.A.; Paniagua, C.E.S.; Alberto, E.A.; Clara, M.; Starling, V.M.; Agüera, A.; Trovó, A.G. A critical review of trends in advanced oxidation processes for the removal of benzophenone-3, fipronil, and propylparaben from aqueous matrices: Pathways and toxicity changes. J. Water Process Eng. 2022, 49, 102973. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Howsawkeng, J.; Watts, R.J.; Washington, D.L.; Teel, A.L.; Hess, T.F.; Crawford, R.L. Evidence for simultaneous abiotic-biotic oxidations in a microbial-Fenton’s system. Environ. Sci. Technol. 2001, 35, 2961–2966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Klamerth, N.; Chelme-Ayala, P.; Gamal El-Din, M. Comparison of Nitrilotriacetic Acid and [S,S]-Ethylenediamine-N,N’-disuccinic Acid in UV-Fenton for the Treatment of Oil Sands Process-Affected Water at Natural pH. Environ. Sci. Technol. 2016, 50, 10535–10544. [Google Scholar] [CrossRef]
- Mejri, A.; Soriano-Molina, P.; Miralles-Cuevas, S.; Trabelsi, I.; Sánchez Pérez, J.A. Effect of liquid depth on microcontaminant removal by solar photo-Fenton with Fe(III):EDDS at neutral pH in high salinity wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28071–28079. [Google Scholar] [CrossRef]
- Trovó, A.G.; de Paiva, V.A.B.; Machado, A.E.H.; de Oliveira, C.A.; Santos, R.O. Degradation of the antibiotic chloramphenicol by photo-Fenton process at lab-scale and solar pilot plant: Kinetic, toxicity and inactivation assessment. Sol. Energy 2013, 97, 596–604. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; García Sánchez, J.L.; Alfano, O.M.; Conte, L.O.; Malato, S.; Sánchez Pérez, J.A. Mechanistic modeling of solar photo-Fenton process with Fe3+-EDDS at neutral pH. Appl. Catal. B Environ. 2018, 233, 234–242. [Google Scholar] [CrossRef]
- Ruales-Lonfat, C.; Barona, J.F.; Sienkiewicz, A.; Vélez, J.; Benítez, L.N.; Pulgarín, C. Bacterial inactivation with iron citrate complex: A new source of dissolved iron in solar photo-Fenton process at near-neutral and alkaline pH. Appl. Catal. B Environ. 2016, 180, 379–390. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; García Sánchez, J.L.; Malato, S.; Pérez-Estrada, L.A.; Sánchez Pérez, J.A. Effect of volumetric rate of photon absorption on the kinetics of micropollutant removal by solar photo-Fenton with Fe3+-EDDS at neutral pH. Chem. Eng. J. 2018, 331, 84–92. [Google Scholar] [CrossRef]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Sacco, O.; Guida, M.; Rizzo, L. Comparison between heterogeneous and homogeneous solar driven advanced oxidation processes for urban wastewater treatment: Pharmaceuticals removal and toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- USP Technologies Titanium Oxalate (Spectrophotometric). 2015. Available online: https://www.h2o2.com/technical-library/analytical-methods/default.aspx?pid=71&name=Titanium-Oxalate-Spectrophotometric (accessed on 22 June 2020).
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H. (Eds.) Standard Methods for Examination of Water & Wastewater, 21st ed.; Amer Public Health Assn: Washington, DC, USA, 2005. [Google Scholar]
- Gomes Júnior, O.; Batista, L.L.; Ueira-Vieira, C.; Sousa, R.M.F.; Starling, M.C.V.M.; Trovó, A.G. Degradation mechanism of fipronil and its transformation products, matrix effects and toxicity during the solar/photo-Fenton process using ferric citrate complex. J. Environ. Manag. 2020, 269, 110756. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.R.; Guimarães, R.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.M.; Trovó, A.G. Reducing toxicity and antimicrobial activity of a pesticide mixture via photo-Fenton in different aqueous matrices using iron complexes. Sci. Total Environ. 2020, 740, 140152. [Google Scholar] [CrossRef] [PubMed]




| Parameter | FeCit | FeEDDS | FeEDTA | FeNTA |
|---|---|---|---|---|
| k (min−1) | 0.10 | 0.036 | 0.051 | 0.063 |
| R2 | 0.96 | 0.97 | 0.99 | 0.99 |
| t1/2 (min) | 6.6 | 19 | 14 | 11 |
| CostIron (USD m−3) | 5.6 | 5.6 | 5.6 | 5.6 |
| CostLigand (USD m−3) | 13 | 321 | 7.7 | 4.5 |
| CostOxidant (USD m−3) | 1.4 | 0.72 | 2.8 | 2.8 |
| CostTotal (USD m−3) | 20 | 327 | 16 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marson, E.O.; Ricardo, I.A.; Paniagua, C.E.S.; Malta, S.M.; Ueira-Vieira, C.; Starling, M.C.V.M.; Sánchez Pérez, J.A.; Trovó, A.G. Effect of Iron Complex Source on MWWTP Effluent Treatment by Solar Photo-Fenton: Micropollutant Degradation, Toxicity Removal and Operating Costs. Molecules 2022, 27, 5521. https://doi.org/10.3390/molecules27175521
Marson EO, Ricardo IA, Paniagua CES, Malta SM, Ueira-Vieira C, Starling MCVM, Sánchez Pérez JA, Trovó AG. Effect of Iron Complex Source on MWWTP Effluent Treatment by Solar Photo-Fenton: Micropollutant Degradation, Toxicity Removal and Operating Costs. Molecules. 2022; 27(17):5521. https://doi.org/10.3390/molecules27175521
Chicago/Turabian StyleMarson, Eduardo O., Ivo A. Ricardo, Cleiseano E. S. Paniagua, Serena M. Malta, Carlos Ueira-Vieira, Maria Clara V. M. Starling, José Antonio Sánchez Pérez, and Alam G. Trovó. 2022. "Effect of Iron Complex Source on MWWTP Effluent Treatment by Solar Photo-Fenton: Micropollutant Degradation, Toxicity Removal and Operating Costs" Molecules 27, no. 17: 5521. https://doi.org/10.3390/molecules27175521