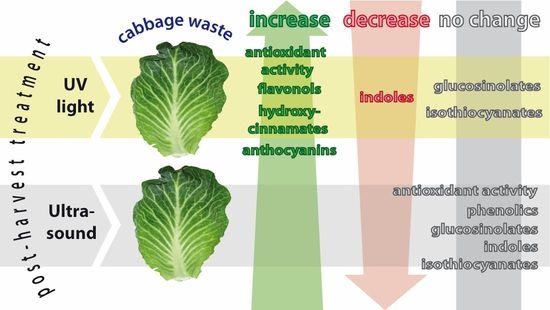
Effects of Post-Harvest Elicitor Treatments with Ultrasound, UV- and Photosynthetic Active Radiation on Polyphenols, Glucosinolates and Antioxidant Activity in a Waste Fraction of White Cabbage (Brassica oleracea var. capitata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Elicitor Treatment
2.1.1. Ultrasound Treatment
2.1.2. Irradiation Treatment

2.2. Sample Preparation
2.3. Chemical Analysis
2.3.1. Chemicals
2.3.2. Determination of Phenolic Compounds
2.3.3. Determination of Antioxidant Activity and Profiling of Antioxidants
2.3.4. Determination of Glucosinolates and Their Degradation Products
2.3.5. Test of Possible Temperature Effect
2.4. Experimental Design and Statistics
3. Results
3.1. Phenolic Compounds
3.2. Glucosinolates and Their Hydrolysis Products
3.3. Antioxidant Activity
4. Discussion
4.1. Phenolic Compounds
4.2. Glucosinolates and Their Autolysis Products
4.3. Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| UV | ultraviolet |
| PAR | photosynthetic active radiation |
| PAL | phenylalanine ammonia-lyase |
| PZT | lead zirconate titanate |
| PE | polyethene |
| ABTS | 2,2-azinobis-(ethyl-2,3-dihydrobenzothiazoline-6-sulphonic acid) diammonium salt |
| TE | Trolox equivalents |
| GLS | glucosinolate(s) |
| GTL | glucotropaeolin |
| ITC | isothiocyanate |
| 3-MSP-ITC | 3-(methylsulfonyl)propyl isothiocyanate |
| DS-GLS | desulfo-derivatives (of glucosinolates) |
References
- Løes, A.K.; Adler, S.; Seljåsen, R.; Carvajal, A.; Tveit, G.M.; Slizyte, R.; Honkapaa, K.; Rommi, K. Food co-streams for innovative food and feed products. In Proceedings of the Nordic Association for Agricultural Science (NJF) 25th Congress Nordic View to Sustainable Rural Development, Riga, Latvia, 16–18 June 2015. [Google Scholar]
- Schreiner, M.; Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and post-harvest elicitors. Crit. Rev. Plant Sciences. 2006, 25, 267–278. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Hang, Y.D.; Downing, D.L.; Stamer, J.R.; Splittstoesser, D.F. Wastes generated in the manufacture of sauerkraut. J. Milk Food Technol. 1972, 35, 432–435. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Schreiner, M. Vegetable crop management strategies to increase the quantity of phytochemicals. Eur. J. Nutr. 2005, 44, 85–94. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The use of controlled post-harvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Hasan, S.M.M.; Angers, P.; Arul, J. UV-B radiation hormesis in broccoli florets: Glucosinolates and hydroxy-cinnamates are enhanced by UV-B in florets during storage. Postharvest Biol. Technol. 2020, 168, 111278. [Google Scholar] [CrossRef]
- Casajús, V.; Civello, P.; Martínez, G.; Howe, K.; Fish, T.; Yang, Y.; Thannhauser, T.; Li, L.; Lobato, M.G. Effect of continuous white light illumination on glucosinolate metabolism during post-harvest storage of broccoli. LWT-Food Sci. Technol. 2021, 145, 111302. [Google Scholar] [CrossRef]
- Kanazawa, K.; Hashimoto, T.; Yoshida, S.; Sungwon, P.; Fukuda, S. Short photoirradiation induces flavonoid synthesis and increases its production in post-harvest vegetables. J. Agric. Food Chem. 2012, 60, 4359–4368. [Google Scholar] [CrossRef]
- Harbaum-Piayda, B.; Palani, K.; Schwarz, K. Influence of post-harvest UV-B treatment and fermentation on secondary plant compounds in white cabbage leaves. Food Chem. 2016, 197, 47–56. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Alcazar, J.; Yang, T.; Luo, Y.; Wang, Q.; Chen, P. Effect of preharvest CaCl2 spray and post-harvest UV-B radiation on storage quality of broccoli microgreens, a richer source of glucosinolates. J. Food Compos. Anal. 2018, 67, 55–62. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- da Silva, J.A.T.; Dobránszki, J. Sonication and ultrasound: Impact on plant growth and development. Plant Cell Tissue Organ. Culture. 2014, 117, 131–143. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L. Elicitor-like effects of low-energy ultrasound on plant (Panax ginseng) cells: Induction of plant defense responses and secondary metabolite production. Appl. Microbiol. Biotechnol. 2002, 59, 51–57. [Google Scholar] [CrossRef]
- Tůmová, L.; Tůma, J.; Hendrychova, H. Effect of ultrasound on the isoflavonoid production in Genista tinctoria L. suspension cultures. Pharmacogn. Mag. 2014, 10, S425–S429. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. The Background. In Plant Propagation by Tissue Culture: Volume 1; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Lens, P.; Virkutyte, J. Low-frequency ultrasound in biotechnology: State of the art. Trends Biotechnol. 2009, 27, 298–306. [Google Scholar] [CrossRef]
- Yu, J.; Engeseth, N.J.; Feng, H. High intensity ultrasound as an abiotic elicitor—Effects on antioxidant capacity and overall quality of romaine lettuce. Food Bioprocess Technol. 2016, 9, 262–273. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. The effects of ultrasound-assisted freezing on the freezing time and quality of broccoli (Brassica oleracea L. var. botrytis L.) during immersion freezing. Int. J. Refrigeration. 2014, 41, 82–91. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Piasek, A.; Bartoszek, A.; Namieśnik, J. The optimisation of analytical parameters for routine profiling of antioxidants in complex mixtures by HPLC coupled post-column derivatisation. Phytochem. Analysis. 2011, 22, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namieśnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography–diode array–electrospray ionisation mass spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr. A 2013, 1278, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Pilipczuk, T.; Dawidowska, N.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. Simultaneous determination of indolic compounds in plant extracts by solid-phase extraction and high-performance liquid chromatography with UV and fluorescence detection. Food Anal. Methods 2015, 8, 2169–2177. [Google Scholar] [CrossRef]
- Pilipczuk, T.; Kusznierewicz, B.; Chmiel, T.; Przychodzeń, W.; Bartoszek, A. Simultaneous determination of individual isothiocyanates in plant samples by HPLC-DAD-MS following SPE and derivatisation with N-acetyl-l-cysteine. Food Chem. 2017, 214, 587–596. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Estrada-Ortiz, E.; Gómez-Merino, F.C.; Becker, C.; Krumbein, A.; Schwarz, D. Flavonoid, nitrate and glucosinolate concentrations in Brassica species are differentially affected by photosynthetically active radiation, phosphate and phosphite. Front. Plant Sci. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Uddin, M.R.; Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.-J.; Nou, I.-S.; Lee, J.; Kim, H.R.; Park, S.U. Accumulation of anthocyanin and related genes expression during the development of cabbage seedlings. Process Biochem. 2014, 49, 1084–1091. [Google Scholar] [CrossRef]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef]
- Ries, G.; Heller, W.; Puchta, H.; Sandermann, H.; Seidlitz, H.K.; Hohn, B. Elevated UV-B radiation reduces genome stability in plants. Nature 2000, 406, 98–101. [Google Scholar] [CrossRef]
- Darré, M.; Valerga, L.; Araque, L.C.O.; Lemoine, M.L.; Demkura, P.V.; Vicente, A.R.; Concellón, A. Role of UV-B irradiation dose and intensity on colour retention and antioxidant elicitation in broccoli florets (Brassica oleracea var. Italica). Postharvest Biol. Technol. 2017, 128, 76–82. [Google Scholar] [CrossRef]
- Lercari, B.; Sodi, F.; Sbrana, C. Comparison of photomorphogenic responses to UV light in red and white cabbage (Brassica oleracea L.). Plant Physiol. 1989, 90, 345–350. [Google Scholar] [CrossRef]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.M.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Non-destructive optical sensing of flavonols and chlorophyll in white head cabbage (Brassica oleracea L. var. capitata sub var. alba) grown under different nitrogen regimes. J. Agric. Food Chem. 2016, 64, 85–94. [Google Scholar] [CrossRef]
- Mageney, V.; Neugart, S.; Albach, D. A guide to the variability of flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef]
- Rybarczyk-Plonska, A.; Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.K.; Wold, A.B. Glucosinolates in broccoli (Brassica oleracea L. var. italica) as affected by post-harvest temperature and radiation treatments. Postharvest Biol. Technol. 2016, 116, 16–25. [Google Scholar] [CrossRef]
- Leasure, C.D.; Chen, Y.-P.; He, Z.-H. Enhancement of indole-3-acetic acid photodegradation by vitamin B6. Mol. Plant. 2013, 6, 1992–1995. [Google Scholar] [CrossRef]
- Reifenrath, K.; Müller, C. Species-specific and leaf-age dependent effects of ultraviolet radiation on two Brassicaceae. Phytochemistry 2007, 68, 875–885. [Google Scholar] [CrossRef]
- Kołodziejski, D.; Piekarska, A.; Hanschen, S.H.; Pilipczuk, T.; Tietz, F.; Kusznierewicz, B.; Bartoszek, A. Relationship between conversion rate of glucosinolates to isothiocyanates/indoles and genotoxicity of individual parts of Brassica vegetables. Eur. Food Res. Technol. 2019, 245, 383–400. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical composition and biological activities of differently pigmented cabbage (Brassica oleracea var. capitata) and cauliflower (Brassica oleracea var. botrytis) varieties. J. Sci. Food Agric. 2019, 99, 5499–5507. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Bączek-Kwinta, R.; Bartoszek, A.; Piekarska, A.; Huk, A.; Manikowska, A.; Antonkiewicz, J.; Namieśnik, J.; Konieczka, P. The dose-dependent influence of zinc and cadmium contamination of soil on their uptake and glucosinolate content in white cabbage (Brassica oleracea var. capitata f. alba). Environ. Toxicol. Chem. 2012, 31, 2482–2489. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Bartoszek, A.; Kusznierewicz, B.; Antonkiewicz, J. Physiological response of plants and cadmium accumulation in heads of two cultivars of white cabbage. J. Elementol. 2011, 16, 355–364. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. Effect of cooking on the contents of glucosinolates and their degradation products in selected Brassica vegetables. J. Funct. Foods 2016, 23, 412–422. [Google Scholar] [CrossRef]




| Treatment Codes | Pre-Treatment | 2nd Treatm. | Main Irradiation Treatment | Post-Treatment | Irradiation Energy (kJ m−2) | Sonic Energy | |||
|---|---|---|---|---|---|---|---|---|---|
| Radiation | Sonication | Radiation | UVB + UVA | PAR | Total | (kJ∙l−1 Water) | |||
| TLD-840 * | DL510H ¤ | TLD-840 | UVB-313 EL | TLD-840 | 250–400 nm | 400–700 nm | 250–700 nm | 35kHz | |
| Control | - | - | - | - | - | 0 | 0 | 0 | 0 |
| S2 | - | 2 min | - | - | - | 0 | 0 | 0 | 15 |
| S4 | - | 4 min | - | - | - | 0 | 0 | 0 | 31 |
| S8 | - | 8 min | - | - | - | 0 | 0 | 0 | 61 |
| PAR | 2 h | - | 10 h | - | 2 h | 17 | 497 | 514 | 0 |
| UVlow | - | - | - | 10 h | - | 59 | 18 | 77 | 0 |
| PAR + UVlow ** | 2 h | - | Both 10 h | Both 10 h | 2 h | 59 | 435 | 494 | 0 |
| PAR + UVhigh *** | 2 h | - | Both 10 h | Both 10 h | 2 h | 99 | 486 | 585 | 0 |
| PAR + UVhigh *** + S8 | 2 h | 8 min | - | 10 h | 2 h | 99 | 486 | 585 | 61 |
| Peak | tR [min] | λmax (nm) | MS (+) | MS (−) | Mw | Putative Identification |
|---|---|---|---|---|---|---|
| 1 | 10.6 | 300sh, 330 | 365 [M + Na]+ | 341 [M − H]− | 342 | Caffeoyl hexoside |
| 2 | 12.2 | 255, 350 | 811 [M + Na]+ | 787 [M − H]− | 788 | Quercetin 3-diglucoside-7-glucoside |
| 3 | 12.6 | 315 | 349 [M + Na]+ | 325 [M − H]− | 326 | Coumaroyl hexoside |
| 4 | 12.9 | 266, 345 | 795 [M +Na]+ | 771 [M − H]− | 772 | Kaempferol 3-diglucoside-7-glucoside |
| 5 | 13.2 | 315 | 349 [M + Na]+ | 325 [M − H]− | 326 | Coumaroyl hexoside |
| 6 | 13.5 | 300sh, 330 | 379 [M + Na]+ | 355 [M − H]− | 356 | Feruloyl hexoside |
| 7 | 13.9 | 330 | 409 [M + Na]+ | 385 [M − H]− | 386 | Sinapoyl hexoside |
| 8 | 20.9 | 330 | - | 753 [M − H]− | 754 | Disinapoylgentiobioside |
| 9 | 21.6 | 330 | - | 959 [M − H]− | 960 | Trisinapoylgentiobioside |
| Desulfoglucosinolates (DS-GLS) | ||||||
| GIB | 4.9 | 229 | 366 [M + Na]+ | 378 [M + Cl]− | 343 | Desulfo-glucoiberin |
| GRA | 5.8 | 229 | 380 [M + Na]+ | 392 [M + Cl]− | 357 | Desulfo-glucoraphanin |
| SIN | 6.9 | 229 | 302 [M + Na]+ | 314 [M + Cl]− | 279 | Desulfo-sinigrin |
| GAL | 11.4 | 229 | 394 [M + Na]+ | 406 [M + Cl]− | 371 | Desulfo-glucoalyssin |
| GBS | 21.7 | 220, 280 | 391 [M + Na]+ | 403 [M + Cl]− | 368 | Desulfo-glucobrassicin |
| neoGBS | 25.4 | 220, 280 | 421 [M + Na]+ | 433 [M + Cl]− | 398 | Desulfo-neoglucobrassicin |
| Conjugates of Isothiocyanates with N-acetyl-l-cysteine (ITC-NAC) | ||||||
| 3-MSP-ITC | 5.5 | 216, 250, 270 | 327 [M + H]+ | - | 326 | 3-(Methylsulphinyl)propyl-ITC-NAC |
| SFN | 6.4 | 216, 250, 270 | 341 [M + H]+ | - | 340 | Sulforaphane-NAC |
| AITC | 8.4 | 216, 250, 270 | 263 [M + H]+ | - | 262 | Allyl-ITC-NAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seljåsen, R.; Kusznierewicz, B.; Bartoszek, A.; Mølmann, J.; Vågen, I.M. Effects of Post-Harvest Elicitor Treatments with Ultrasound, UV- and Photosynthetic Active Radiation on Polyphenols, Glucosinolates and Antioxidant Activity in a Waste Fraction of White Cabbage (Brassica oleracea var. capitata). Molecules 2022, 27, 5256. https://doi.org/10.3390/molecules27165256
Seljåsen R, Kusznierewicz B, Bartoszek A, Mølmann J, Vågen IM. Effects of Post-Harvest Elicitor Treatments with Ultrasound, UV- and Photosynthetic Active Radiation on Polyphenols, Glucosinolates and Antioxidant Activity in a Waste Fraction of White Cabbage (Brassica oleracea var. capitata). Molecules. 2022; 27(16):5256. https://doi.org/10.3390/molecules27165256
Chicago/Turabian StyleSeljåsen, Randi, Barbara Kusznierewicz, Agnieszka Bartoszek, Jørgen Mølmann, and Ingunn M. Vågen. 2022. "Effects of Post-Harvest Elicitor Treatments with Ultrasound, UV- and Photosynthetic Active Radiation on Polyphenols, Glucosinolates and Antioxidant Activity in a Waste Fraction of White Cabbage (Brassica oleracea var. capitata)" Molecules 27, no. 16: 5256. https://doi.org/10.3390/molecules27165256