Structural Characterization of a Polygonatum cyrtonema Hua Tuber Polysaccharide and Its Contribution to Moisture Retention and Moisture-Proofing of Porous Carbohydrate Material
Abstract
:1. Introduction
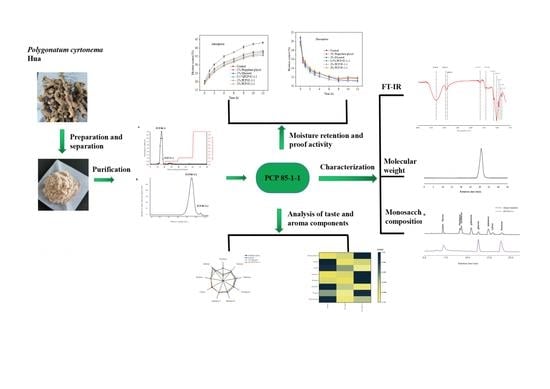
2. Results and Discussion
2.1. Extraction and Comparison of Ethanol Precipitated Fractions from Water Extracts
2.2. Moisture Absorption and Moisture Retention Properties of PCP
2.2.1. Moisture Absorption Properties
2.2.2. Moisture Retention Properties
2.3. Purification, Chemical, and Conformational Characteristics of PCP 85−1−1
2.4. FT-IR Spectra Characteristics of PCP 85−1−1
2.5. Moisture-Proofing and Moisture Retention Experiment with Tobacco Shreds with PCP 85−1−1
2.5.1. Moisture-Proofing Experiment
2.5.2. Moisture Retention Experiment
2.5.3. Calculation of MRI and MPI
2.6. Analysis of Taste and Aroma Components
2.6.1. Electronic Tongue Evaluation
2.6.2. Volatile Compound Contents
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Extraction and Fractionation Procedure
3.3. Moisture Absorption and Moisture Retention Properties of PCP
3.3.1. Moisture Absorption Properties
3.3.2. Moisture Retention Properties
3.4. Separation and Purification of PCP 85
3.5. Monosaccharide Components Analysis of PCP 85−1−1
3.6. Molecular Weight Analysis of PCP 85−1−1
3.7. FT-IR Spectroscopic Analysis of PCP 85−1−1
3.8. Evaluation of Adsorption and Desorption Processes of Tobacco Shreds
3.8.1. Moisture-Proofing and Moisture Retention of Tobacco Shreds with PCP 85−1−1
3.8.2. Calculation of MRI and MPI
3.9. Aroma Analysis
3.9.1. Detection and Analysis by Electronic Tongue
3.9.2. GC-MS Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, M.; Zhang, G.; Liu, C.; Li, B.; Zhang, K.; Lv, B. Effect of konjac glucomannan on hygroscopic characteristics of cut tobacco. Food Mach. 2019, 35, 204–209. [Google Scholar] [CrossRef]
- Lei, S.; Liu, Y.M.; Li, Y.D.; Jiang, J.X.; Zeng, Z.C.; Wang, Y.H.; Lin, C.; Wang, X.Q.; Yang, Q.Y. Moisture retention properties of Polygonatum polysaccharide in tobacco and its mechanism. Food Mach. 2019, 35, 49–54. [Google Scholar] [CrossRef]
- Lei, S.; Liu, Y.M.; Li, Y.D.; Jiang, J.X.; Zeng, Z.C.; Wang, Y.H.; Lin, C.; Wang, X.Q.; Yang, Q.Y. Effect of natural polysaccharide on moisture retention capacity and moisture kinetics of tobacco. Food Mach. 2020, 36, 28–32+38. [Google Scholar] [CrossRef]
- Yan, H.; Cai, B.; Cheng, Y.; Guo, G.; Li, D.; Yao, X.; Ni, X.; Phillips, G.O.; Fang, Y.; Jiang, F. Mechanism of lowering water activity of konjac glucomannan and its derivatives. Food Hydrocoll. 2012, 26, 383–388. [Google Scholar] [CrossRef]
- Wang, L.; Cardenas, R.B.; Watson, C. An isotope dilution ultra high performance liquid chromatography-tandem mass spectrometry method for the simultaneous determination of sugars and humectants in tobacco products. J. Chromatogr. A 2017, 1514, 95. [Google Scholar] [CrossRef]
- Chen, Z.F.; Lu, C.T.; Sun, Z.T.; Dai, J.G.; Qu, Z.; Ma, J. Synthesis of two hydrophilic polyhydroxy compounds and their application in cigarette production. Acta Tab. Sin. 2014, 20, 36–41. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.T.; Tian, Q.H.; Xiao, L.X.; Zeng, Z.; Cai, X.T.; Yan, J.Z.; Li, Q.Y. Microwave-Assisted Degradation of Polysaccharide from Polygonatum sibiricum and Antioxidant Activity. J. Food Sci. 2019, 84, 754–761. [Google Scholar] [CrossRef]
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C. A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides. Molecules 2018, 23, 1170. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y. Research Progress on the active components and pharmacological action of Polygonatum cyrtonema. Fujian Agric. Sci. Technol. 2021, 52, 39–44. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, Y.; Li, Y.; Liu, Y. Analysis of amino acid composition and nutritional value of Polygonatum cyrtonema “Li Jing No. 1”. J. Chin. Med. Mater. 2021, 44, 2147–2150. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Wang, Y.; Zhang, X.; Jia, H.; Guo, L.; Huang, L.; Gao, W. Comparative studies on characterization, saccharide mapping and antiglycation activity of polysaccharides from different Polygonatum ssp. J. Pharm. Biomed. Anal. 2020, 186, 113243. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Y.; Bao, Y.; Wang, M.; Hou, M. Saponins from the rhizomes of Polygonatum nodosum Hua and their chemotaxonomic significance. Biochem. Syst. Ecol. 2021, 98, 104308. [Google Scholar] [CrossRef]
- Horng, C.T.; Huang, J.K.; Wang, H.Y.; Huang, C.C.; Chen, F.A. Antioxidant and antifatigue activities of Polygonatum Alte-lobatum Hayata rhizomes in rats. Nutrients 2014, 6, 5327–5337. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Thakur, K.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J. Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 114, 317–323. [Google Scholar] [CrossRef]
- Long, T.; Liu, Z.; Shang, J.; Zhou, X.; Yu, S.; Tian, H.; Bao, Y. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-kappαβ signaling pathways. Int. J. Biol. Macromol. 2018, 111, 813–821. [Google Scholar] [CrossRef]
- Li, R.; Tao, A.; Yang, R.; Fan, M.; Zhang, X.; Du, Z.; Shang, F.; Xia, C.; Duan, B. Structural characterization, hypoglycemic effects and antidiabetic mechanism of a novel polysaccharides from Polygonatum kingianum Coll. et Hemsl. Biomed. Pharmacother. 2020, 131, 110687. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, Z.; Zhong, C.; Zhang, X.; Bao, Y. Immunomodulatory effects of a polysaccharide from Solanum nigrum Linne through TLR4-MyD88 signaling pathway. Int. Immunopharmacol. 2020, 88, 106973. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, H.; Li, Y.; Liu, Y.; Dai, W.; Fang, J.; Cao, C.; Die, Y.; Liu, Q.; Wang, C.; et al. Physicochemical properties and immunological activities of polysaccharides from both crude and wine-processed Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 143, 255–264. [Google Scholar] [CrossRef]
- Lin, C.; Cui, H.; Wang, X.; Wang, H.; Xia, S.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X. Regulating water binding capacity and improving porous carbohydrate matrix’s humectant and moisture proof functions by mixture of sucrose ester and Polygonatum sibiricum polysaccharide. Int. J. Biol. Macromol. 2020, 147, 667–674. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Zhu, D.; He, Y.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Gong, G.; Dang, T.; Deng, Y.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Li, J.E.; Wang, W.J.; Zheng, G.D.; Li, L.Y. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int. J. Biol. Macromol. 2017, 95, 719–724. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yan, B.; Yao, C.; Chen, X.; Li, L.; Wu, Y.; Song, Z.; Song, S.; Zhang, Z.; Luo, P. Oligosaccharides from Polygonatum Cyrtonema Hua: Structural characterization and treatment of LPS-induced peritonitis in mice. Carbohydr. Polym. 2021, 255, 117392. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Luo, L.; Zhou, Z.; Wang, Y.; Gao, T.; Yang, L.; Peng, T.; Wu, M. Structures of fructan and galactan from Polygonatum cyrtonema and their utilization by probiotic bacteria. Carbohydr. Polym. 2021, 267, 118219. [Google Scholar] [CrossRef]
- Chou, C.H.; Sung, T.J.; Hu, Y.N.; Lu, H.Y.; Yang, L.C.; Cheng, K.C.; Lai, P.S.; Hsieh, C.W. Chemical analysis, moisture-preserving, and antioxidant activities of polysaccharides from Pholiota nameko by fractional precipitation. Int. J. Biol. Macromol. 2019, 131, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Ma, Y.; Huang, Y.; Li, S.; Xu, H.; Su, E. Current advances in the biosynthesis of hyaluronic acid with variable molecular weights. Carbohydr. Polym. 2021, 269, 118320. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Shao, J.; Han, L.; Lv, R.; Sun, P. Separation, preliminary characterization, and moisture-preserving activity of polysaccharides from Ulva fasciata. Int. J. Biol. Macromol. 2015, 72, 924–930. [Google Scholar] [CrossRef]
- Chen, W.; Wang, N.; Zhang, M. Cellulase degradation and physicochemical propertyanalysis of oat polysaccharide. China Food Addit. 2014, 2, 159–163. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.H.; Meng, Y.W.; Yang, M.; He, K.Z. Structure of polysaccharide from Polygonatum cyrtonema hua and the antiherpetic activity of its hydrolyzed fragments. Antivir. Res. 2004, 63, 183–189. [Google Scholar] [CrossRef]
- Liu, X.X.; Wan, Z.J.; Shi, L.; Lu, X.X. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema hua. Carbohydr. Polym. 2011, 83, 737–742. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107 Pt A, 796–802. [Google Scholar] [CrossRef]
- Lv, X.; Chen, D.; Yang, L.; Zhu, N.; Li, J.; Zhao, J.; Hu, Z.; Wang, F.J.; Zhang, L.W. Comparative studies on the immunoregulatory effects of three polysaccharides using high content imaging system. Int. J. Biol. Macromol. 2016, 86, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.Y.; Ren, T.B.; Feng, X.Y.; Song, J.; Ji, X.M. Response Surface Methodology for Optimization Tobacco Leaf Polysaccharides Phosphorylation Process and Evaluation of Its Humectant Properties. Fine Chem. 2018, 35, 2067–2071. [Google Scholar] [CrossRef]
- Huang, F.F.; Jiang, J.; Li, X.Q.; Zhang, Y.; Yang, J.; Zhang, A.Q.; Sun, P.L. Ultrasonic Extraction Technique for Polysaccharide from Wedelia prostrate and MoistureRetentivity of Polysaccharide in Cigarettes. Tob. Sci. Technol. 2014, 2, 44–48. [Google Scholar] [CrossRef]
- Feng, X.L.; Fang, H.; Liu, J.X.; Sun, Y.; Zhou, L.W. Preparation and properties of schizophyllan polysaccharide phosphorylated derivative. China Surfactant Deterg. Cosmet. 2017, 11, 637–640. [Google Scholar] [CrossRef]
- Yin, C.; Xu, Z.; Shu, J.; Wang, H.; Li, Y.; Sun, W.F.; Zhou, Z.L. Study on the effect of potassium lactate additive on the combustion behavior and mainstream smoke of cigarettes. J. Therm. Anal. Calorim. 2014, 115, 1733–1751. [Google Scholar] [CrossRef]
- Zha, Q.; Moldoveanu, S. The influence of cigarette moisture to the chemistry of particulate phase smoke of a common commercial cigarette. Beiträge Zur Tab. Contrib. Tob. Res. 2004, 21, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.T.; Liu, Y.; Liu, S.; Bai, X.L.; Gao, C.C.; Zhao, M.Y.; Hu, J. Factors influencing moisture retentivity of tobacco leaf. Tob. Sci. Technol. 2011, 8, 62–67. [Google Scholar] [CrossRef]
- Talhout, R.; Opperhuizen, A.; Amsterdam van, J.G.C. Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food Chem. Toxicol. 2006, 44, 1789–1798. [Google Scholar] [CrossRef]
- Gaworski, C.L.; Oldham, M.J.; Coggins, C.R. Toxicological considerations on the use of propylene glycol as a humectant in cigarettes. Toxicology 2010, 269, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chu, G.L.; He, L.; Hu, J.L.; Luo, H.T. Analysis of pyrolytic properties of polysaccharides and its application in cigarette. Food Mach. 2020, 36, 217–222. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, P.H.; Lu, W.C.; Chan, Y.J.; Ko, W.C.; Jung, C.C.; Huynh, D. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, N.; Xue, X.; Li, Q.; Sun, D.; Zhao, Z. Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 160, 688–694. [Google Scholar] [CrossRef]
- Yin, C.; Xu, Z.; Shu, J.; Li, Y.; Sun, W.; Zhou, Z.; Chen, M.; Zhong, F. Influence of Physicochemical Characteristics on the Effective Moisture Diffusivity in Tobacco. Int. J. Food Prop. 2015, 18, 690–698. [Google Scholar] [CrossRef]
- Lou, J.; Sha, Y.; Wu, D.; Zhu, Y.; Zhang, Q.; Liu, B.; Yan, Y. A Test Method for Moisture Retention Property of Tobacco. CN Patent CN105842103A, 25 September 2018. Available online: https://patents.google.com/patent/CN105842103A/zh (accessed on 2 February 2022).
- Gao, B.; Hu, X.; Li, R.; Zhao, Y.; Tu, Y.; Zhao, Y. Screening of characteristic umami substances in preserved egg yolk based on the electronic tongue and UHPLC-MS/MS. LWT 2021, 152, 112396. [Google Scholar] [CrossRef]
- National Tobacco Quality Supervision and Inspection Center, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China. Cigarette-Determination of Total and Nicotine-Free Dry Particulate Matter Using a Routine Analytical Smoking Machine. 2004. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SCSF00027319&DbName=SCSF (accessed on 1 March 2005).







| PCP 20 | PCP 40 | PCP 60 | PCP 85 | |
|---|---|---|---|---|
| Polysaccharide yield (%) | 1.34 ± 0.88 a | 5.32 ± 0.54 c | 2.47 ± 0.61 b | 15.43 ± 1.21 d |
| Purity (%) | 62.44 ± 1.64 d | 56.64 ± 3.57 a | 57.77 ± 1.58 b | 58.18 ± 2.25 c |
| Protein (mg/g) | 3.98 ± 0.0075 c | 3.15 ± 0.10 a | 3.83 ± 0.015 c | 3.45 ± 0.11 b |
| Polyphenol (mg/g) | 5.54 ± 0.21 d | 2.76 ± 0.059 c | 2.28 ± 0.036 b | 1.61 ± 0.001 a |
| Sample | Adsorption | Desorption | ||||||
|---|---|---|---|---|---|---|---|---|
| k | n | R2 | E (%) | k | n | R2 | E (%) | |
| Control | 0.7884 | 0.4148 | 0.9939 | 0.9367 | 0.2727 | 0.8418 | 0.9780 | 1.5053 |
| 1% Propylene glycol | 0.7497 | 0.4089 | 0.9935 | 0.9820 | 0.2791 | 0.8206 | 0.9794 | 1.5015 |
| 1% Glycerol | 0.7179 | 0.4089 | 0.9947 | 1.0144 | 0.2220 | 0.8876 | 0.9804 | 1.6381 |
| 0.5% PCP 85−1−1 | 0.7860 | 0.3947 | 0.9968 | 0.9496 | 0.2809 | 0.8119 | 0.9796 | 1.4992 |
| 1% PCP 85−1−1 | 0.7061 | 0.4361 | 0.9933 | 1.0179 | 0.2346 | 0.8668 | 0.9770 | 1.6104 |
| 2% PCP 85−1−1 | 0.6694 | 0.4208 | 0.9932 | 1.0789 | 0.2290 | 0.8791 | 0.9800 | 1.6179 |
| Sample | Adsorption | Desorption | ||||
|---|---|---|---|---|---|---|
| EMC (%) | v (%/h) | MRI | EMC (%) | v (%/h) | MPI | |
| Control | 43.07 | 0.88 | 1.95 | 11.79 | 1.88 | 1.67 |
| 1% Propylene glycol | 38.38 | 0.80 | 2.05 | 11.69 | 1.57 | 2.00 |
| 1% Glycerol | 37.18 | 0.84 | 1.98 | 11.25 | 1.50 | 2.07 |
| 0.5% PCP 85−1−1 | 37.86 | 0.83 | 1.95 | 11.16 | 1.56 | 2.01 |
| 1% PCP 85−1−1 | 36.03 | 0.82 | 1.96 | 11.01 | 1.45 | 2.11 |
| 2% PCP 85−1−1 | 35.55 | 0.75 | 2.11 | 12.12 | 1.43 | 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Wang, Y.; Tang, Q.; Zhang, R.; Zhang, D.; Zhu, G. Structural Characterization of a Polygonatum cyrtonema Hua Tuber Polysaccharide and Its Contribution to Moisture Retention and Moisture-Proofing of Porous Carbohydrate Material. Molecules 2022, 27, 5015. https://doi.org/10.3390/molecules27155015
Yu L, Wang Y, Tang Q, Zhang R, Zhang D, Zhu G. Structural Characterization of a Polygonatum cyrtonema Hua Tuber Polysaccharide and Its Contribution to Moisture Retention and Moisture-Proofing of Porous Carbohydrate Material. Molecules. 2022; 27(15):5015. https://doi.org/10.3390/molecules27155015
Chicago/Turabian StyleYu, Ling, Yipeng Wang, Qingjiu Tang, Rongrong Zhang, Danyu Zhang, and Guangyong Zhu. 2022. "Structural Characterization of a Polygonatum cyrtonema Hua Tuber Polysaccharide and Its Contribution to Moisture Retention and Moisture-Proofing of Porous Carbohydrate Material" Molecules 27, no. 15: 5015. https://doi.org/10.3390/molecules27155015