Effect of Different Processing Methods on the Chemical Constituents of Scrophulariae Radix as Revealed by 2D NMR-Based Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Samples Collection and Processing
2.2. Optimization of Extraction Conditions
2.2.1. Selecting the Optimum Extraction Solvent
2.2.2. Optimization of the Solid/Liquid Ratio of the Extraction
2.2.3. Optimum Sample Amount Used per NMR Sample
2.3. Comparison of SR Being Steamed Processing for Different Time
2.3.1. Multivariate Statistical Analysis
2.3.2. Change of Chemical Constituents of SR Steamed for Different Durations
| Feature | δH | δC | Assignment | Feature | δH | δC | Assignment | Feature | δH | δC | Assignment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F115 | 2.72 | 46.66 | aucubin | F121 | 3.02 | 49.38 | aucubin | F124 | 5.05 | 99.01 | aucubin |
| F128 | 5.81 | 131.75 | aucubin | F127 | 5.12 | 107.85 | aucubin | F135 | 6.32 | 142.93 | aucubin |
| F179 | 4.33 | 62.72 | aucubin | F196 | 4.35 | 62.72 | aucubin | F283 | 3.84 | 79.19 | harpagoside |
| F299 | 5.01 | 108.14 | harpagoside | F346 | 2.95 | 56.61 | harpagoside | F372 | 6.16 | 96.43 | harpagoside |
| F408 | 6.47 | 145.71 | harpagoside | F146 | 1.52 | 24.36 | harpagoside | F558 | 2.29 | 47.69 | harpagoside |
| F581 | 2.04 | 47.68 | harpagoside | F773 | 6.55 | 121.43 | harpagoside, cinnamic acid | F225 | 7.63 | 131.07 | harpagoside, cinnamic acid |
| F679 | 7.69 | 148.31 | harpagoside, cinnamic acid | F101 | 4.72 | 101.44 | aucubin, harpagoside | F118 | 1.26 | 26.82 | harpagide |
| F242 | 5.03 | 109.70 | harpagide | F216 | 3.78 | 79.47 | harpagide | F219 | 4.67 | 101.09 | harpagide |
| F279 | 6.37 | 144.22 | harpagide | F254 | 2.57 | 60.43 | harpagide | F273 | 5.73 | 95.26 | harpagide |
| F375 | 1.95 | 48.66 | harpagide | F294 | 1.85 | 48.66 | harpagide | F607 | 1.83 | 48.66 | harpagide |
| F495 | 1.97 | 48.66 | harpagide | F106 | 3.30 | 76.01 | aucubin, harpagoside, harpagide | F268 | 3.31 | 75.64 | aucubin, harpagoside, harpagide |
| F244 | 3.35 | 72.89 | aucubin, harpagoside, harpagide, glucose | F220 | 3.77 | 61.50 | 6-O-methyl-catalpol | F218 | 2.35 | 38.86 | 6-O-methyl-catalpol |
| F229 | 6.40 | 143.71 | 6-O-methyl-catalpol | F207 | 5.08 | 105.85 | 6-O-methyl-catalpol | F520 | 4.18 | 63.00 | 6-O-methyl-catalpol |
| F138 | 4.82 | 101.35 | 6-O-methyl-catalpol | F201 | 5.05 | 97.23 | 6-O-methyl-catalpol | F123 | 4.20 | 62.79 | 6-O-methyl-catalpol |
| F184 | 3.35 | 76.10 | 6-O-methyl-catalpol | F194 | 2.60 | 44.63 | 6-O-methyl-catalpol | F178 | 4.23 | 62.72 | 6-O-methyl-catalpol |
| F111 | 3.48 | 78.90 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol, glucuronic acid | F107 | 3.38 | 79.20 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol, glucose | F108 | 3.37 | 79.39 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol |
| F42 | 3.45 | 79.22 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol, glucuronic acid | F300 | 3.41 | 79.25 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol | F228 | 3.41 | 79.45 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol, glucose |
| F649 | 1.53 | 27.47 | isoleucine | F367 | 3.39 | 78.91 | aucubin, harpagoside, harpagide, 6-O-methyl-catalpol, glucose | F460 | 3.61 | 62.61 | isoleucine |
| F238 | 0.96 | 14.06 | isoleucine | F611 | 1.98 | 39.00 | isoleucine | F224 | 1.03 | 17.53 | isoleucine |
| F159 | 7.18 | 133.57 | tyrosine | F330 | 2.65 | 32.01 | methionine | F1423 | 3.00 | 38.63 | tyrosine |
| F659 | 3.20 | 38.64 | tyrosine | F147 | 6.85 | 118.65 | tyrosine | F248 | 3.88 | 59.18 | tyrosine |
| F339 | 2.05 | 28.38 | glutamine | F357 | 3.01 | 38.65 | tyrosine | F389 | 3.22 | 38.65 | tyrosine |
| F186 | 3.53 | 63.50 | threonine, valine | F261 | 2.09 | 28.39 | glutamine | F145 | 2.07 | 28.41 | glutamine |
| F596 | 1.76 | 43.04 | leucine | F95 | 1.35 | 22.64 | threonine | F235 | 0.99 | 25.00 | leucine |
| F297 | 1.73 | 27.11 | leucine, arginine | F230 | 0.97 | 23.83 | leucine | F637 | 1.66 | 43.03 | leucine |
| F198 | 1.94 | 30.71 | arginine | F350 | 1.67 | 27.09 | arginine | F470 | 3.21 | 43.69 | arginine |
| F40 | 3.83 | 75.61 | sucrose | F213 | 1.90 | 30.67 | arginine | F622 | 3.82 | 62.76 | sucrose |
| F31 | 3.80 | 63.37 | sucrose | F53 | 3.44 | 72.46 | sucrose | F28 | 3.83 | 65.34 | sucrose |
| F19 | 3.76 | 75.90 | sucrose | F55 | 3.78 | 63.39 | sucrose | F18 | 3.54 | 74.19 | sucrose |
| F5 | 3.81 | 65.32 | sucrose, stachyose, raffinose | F2 | 3.66 | 64.87 | sucrose, stachyose | F12 | 3.78 | 65.34 | sucrose, stachyose, raffinose |
| F11 | 5.43 | 95.10 | sucrose, stachyose, raffinose | F6 | 4.18 | 79.84 | sucrose, stachyose, raffinose | F9 | 3.85 | 84.66 | sucrose, stachyose, raffinose |
| F23 | 4.95 | 101.65 | stachyose, raffinose | F65 | 5.41 | 95.23 | sucrose, stachyose, raffinose | F20 | 4.12 | 71.75 | stachyose |
| F25 | 3.67 | 69.01 | stachyose, raffinose | F22 | 4.02 | 69.01 | stachyose, raffinose | F21 | 4.06 | 74.43 | stachyose, raffinose |
| F46 | 3.23 | 77.36 | glucose, glucuronic acid | F56 | 3.71 | 76.20 | sucrose, glucose, glucuronic acid | F51 | 5.21 | 95.42 | glucuronic acid |
| F258 | 3.25 | 77.38 | glucose | F116 | 3.81 | 74.47 | glucose | F54 | 3.48 | 74.80 | glucose |
| F137 | 3.92 | 63.97 | glucose | F103 | 4.58 | 99.25 | β-glucose | F102 | 3.21 | 77.44 | glucose |
| F97 | 4.01 | 66.26 | fructose | F155 | 3.46 | 74.92 | glucose | F57 | 4.03 | 66.26 | fructose |
| F328 | 3.70 | 67.08 | fructose | F44 | 3.81 | 70.65 | fructose | F180 | 3.98 | 79.17 | fructose |
| F41 | 3.71 | 67.08 | fructose | F74 | 3.70 | 66.25 | fructose | F154 | 4.07 | 85.43 | fructose |
| F61 | 3.55 | 65.84 | fructose | F35 | 3.53 | 67.08 | fructose | F321 | 3.51 | 67.08 | fructose |
| F48 | 3.68 | 66.24 | fructose | F68 | 4.08 | 78.69 | fructose | F163 | 3.79 | 70.65 | fructose |
| F71 | 3.53 | 65.82 | fructose | F692 | 3.02 | 41.93 | 4-aminobutyric acid | F199 | 2.32 | 37.41 | 4-aminobutyric acid |
| F175 | 3.01 | 42.37 | 4-aminobutyric acid | F192 | 1.91 | 26.53 | 4-aminobutyric acid | F624 | 2.29 | 32.19 | valine |
| F237 | 1.06 | 20.98 | valine | F232 | 1.02 | 19.63 | valine | F223 | 3.92 | 79.83 | |
| F260 | 3.24 | 56.82 | F386 | 1.94 | 25.75 | F182 | 3.21 | 56.76 | |||
| F546 | 4.05 | 58.63 | F758 | 2.67 | 39.47 | F92 | 4.14 | 61.30 | |||
| F34 | 4.62 | 99.40 | F946 | 2.83 | 39.45 | F975 | 3.12 | 44.34 | |||
| F791 | 3.81 | 60.65 | F52 | 3.73 | 68.65 | F503 | 3.51 | 70.54 | |||
| F473 | 3.86 | 55.36 | F740 | 6.78 | 118.64 | F864 | 2.81 | 39.47 | |||
| F292 | 2.50 | 28.39 | F156 | 2.48 | 28.41 | F309 | 2.41 | 36.86 | |||
| F872 | 6.67 | 123.63 | F176 | 2.40 | 32.70 | F853 | 6.79 | 119.26 | |||
| F90 | 2.38 | 32.74 | F181 | 4.02 | 84.29 | F204 | 2.36 | 32.69 | |||
| F285 | 2.46 | 28.39 | F278 | 5.04 | 110.79 | F642 | 3.59 | 49.68 | |||
| F708 | 2.11 | 32.99 | F288 | 3.59 | 72.65 | F49 | 3.98 | 68.66 | |||
| F36 | 3.85 | 72.91 | F315 | 5.11 | 110.38 | F120 | 3.50 | 74.41 | |||
| F487 | 1.30 | 19.68 | F322 | 4.00 | 86.92 | F500 | 3.67 | 56.35 | |||
| F502 | 4.18 | 85.47 | F354 | 4.24 | 82.63 | F545 | 2.82 | 37.70 | |||
| F505 | 3.82 | 55.44 | F480 | 5.06 | 110.70 | F772 | 3.40 | 58.90 | |||
| F636 | 2.04 | 24.55 | F557 | 4.25 | 84.47 | F1400 | 5.43 | 75.92 | |||
| F405 | 3.25 | 77.58 | F608 | 3.96 | 80.14 | F1430 | 4.97 | 72.81 | |||
| F699 | 2.21 | 32.98 | F616 | 4.10 | 84.97 | F1483 | 5.43 | 74.44 | |||
| F447 | 2.75 | 39.54 | F645 | 4.09 | 84.02 | F117 | 3.51 | 60.15 | |||
| F43 | 3.59 | 77.32 | F646 | 3.91 | 76.55 | F188 | 3.76 | 74.20 | |||
| F723 | 3.42 | 57.74 | F777 | 1.79 | 29.00 | F195 | 3.75 | 89.84 | |||
| F66 | 3.80 | 84.11 | F921 | 2.15 | 23.05 | F739 | 6.56 | 138.61 | |||
| F72 | 4.00 | 73.00 | F745 | 1.91 | 33.03 | F287 | 4.18 | 77.06 | |||
| F89 | 4.08 | 77.78 | F1209 | 5.90 | 128.65 | F457 | 4.06 | 84.65 | |||
| F349 | 2.14 | 16.84 | F13 | 4.06 | 77.06 | F29 | 3.83 | 71.78 | |||
| F32 | 4.16 | 71.74 | F125 | 4.48 | 84.00 | F206 | 4.06 | 79.80 | |||
| F174 | 3.87 | 73.28 | F422 | 3.56 | 63.35 | F750 | 5.43 | 85.29 | |||
| F193 | 3.61 | 66.25 | F845 | 4.29 | 84.69 | F684 | 3.36 | 49.67 | |||
| F256 | 4.10 | 84.45 | F932 | 4.16 | 85.44 |
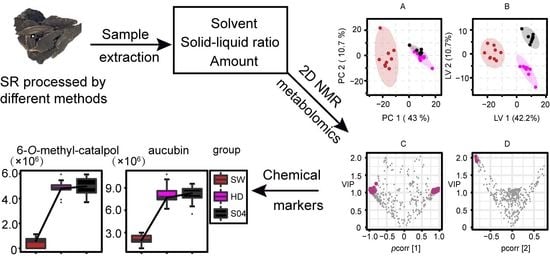
2.4. Comparison of SR Processed by SW, HD, and S04
2.4.1. Multivariate Statistical Analysis
2.4.2. Identification of Differential Metabolites of SW, HD, and S04
| Feature | δH | δC | Assignment | Feature | δH | δC | Assignment | Feature | δH | δC | Assignment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F115 | 2.72 | 46.66 | aucubin | F121 | 3.02 | 49.38 | aucubin | F124 | 5.05 | 99.01 | aucubin |
| F135 | 6.32 | 142.93 | aucubin | F128 | 5.81 | 131.75 | aucubin | F179 | 4.33 | 62.72 | aucubin |
| F127 | 5.12 | 107.85 | aucubin | F685 | 2.27 | 47.68 | harpagoside | F299 | 5.01 | 108.14 | harpagoside |
| F408 | 6.47 | 145.71 | harpagoside | F372 | 6.16 | 96.43 | harpagoside | F346 | 2.95 | 56.61 | harpagoside |
| F146 | 1.52 | 24.36 | harpagoside | F625 | 2.06 | 47.68 | harpagoside | F283 | 3.84 | 79.19 | harpagoside |
| F712 | 7.66 | 148.31 | harpagoside, cinnamic acid | F225 | 7.63 | 131.07 | harpagoside, cinnamic acid | F773 | 6.55 | 121.43 | harpagoside, cinnamic acid |
| F671 | 6.53 | 121.43 | harpagoside, cinnamic acid | F101 | 4.72 | 101.44 | aucubin, harpagoside | F244 | 3.35 | 72.89 | aucubin, harpagoside, glucose |
| F138 | 4.82 | 101.35 | 6-O-methyl-catalpol | F207 | 5.08 | 105.85 | 6-O-methyl-catalpol | F194 | 2.60 | 44.63 | 6-O-methyl-catalpol |
| F123 | 4.20 | 62.79 | 6-O-methyl-catalpol | F184 | 3.35 | 76.10 | 6-O-methyl-catalpol | F229 | 6.40 | 143.71 | 6-O-methyl-catalpol |
| F201 | 5.05 | 97.23 | 6-O-methyl-catalpol | F218 | 2.35 | 38.86 | 6-O-methyl-catalpol | F220 | 3.77 | 61.50 | 6-O-methyl-catalpol |
| F42 | 3.45 | 79.22 | aucubin, harpagoside, 6-O-methyl-catalpol, glucose | F333 | 3.39 | 79.72 | aucubin, harpagoside, 6-O-methyl-catalpol, glucose | F111 | 3.48 | 78.90 | aucubin, harpagoside, 6-O-methyl-catalpol |
| F108 | 3.37 | 79.39 | aucubin, harpagoside, 6-O-methyl-catalpol | F367 | 3.39 | 78.91 | aucubin, harpagoside, 6-O-methyl-catalpol, glucose | F130 | 3.46 | 79.14 | aucubin, harpagoside, 6-O-methyl-catalpol |
| F107 | 3.38 | 79.20 | aucubin, harpagoside, 6-O-methyl-catalpol, glucose | F211 | 3.44 | 79.30 | aucubin, harpagoside, 6-O-methyl-catalpol | F494 | 3.80 | 56.89 | methionine |
| F699 | 2.21 | 32.98 | methionine | F349 | 2.14 | 16.84 | methionine | F330 | 2.65 | 32.01 | methionine |
| F708 | 2.11 | 32.99 | methionine | F659 | 3.20 | 38.64 | tyrosine | F1423 | 3.00 | 38.63 | tyrosine |
| F248 | 3.88 | 59.18 | tyrosine | F103 | 4.58 | 99.25 | β-glucose | F150 | 5.19 | 95.24 | α-glucose |
| F102 | 3.21 | 77.44 | glucose | F50 | 3.73 | 57.21 | F1209 | 5.90 | 128.65 | ||
| F88 | 3.98 | 72.38 | F667 | 5.14 | 98.33 | F125 | 4.48 | 84.00 | |||
| F77 | 3.91 | 74.04 | F1055 | 1.58 | 30.33 | F340 | 1.24 | 17.22 | |||
| F117 | 3.51 | 60.15 | F195 | 3.75 | 89.84 | F514 | 1.94 | 24.72 | |||
| F622 | 3.82 | 62.76 | F47 | 1.35 | 23.18 | F610 | 2.36 | 37.79 | |||
| F723 | 3.42 | 57.74 | F606 | 4.01 | 82.57 | F631 | 4.45 | 105.80 | |||
| F988 | 1.93 | 29.66 | F689 | 7.38 | 132.36 | F741 | 3.13 | 51.58 | |||
| F717 | 1.95 | 29.64 | F727 | 1.99 | 29.63 | F760 | 3.35 | 76.83 | |||
| F761 | 3.26 | 55.60 | F772 | 3.40 | 58.90 | F989 | 4.81 | 101.63 | |||
| F777 | 1.79 | 29.00 | F794 | 3.35 | 55.66 | F800 | 4.02 | 66.89 | |||
| F182 | 3.21 | 56.76 | F503 | 3.51 | 70.54 | F546 | 4.05 | 58.63 |
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Samples Collection and Processing
3.2.1. Fresh SR Raw Material Collection
3.2.2. SR Raw Material Samples Processing
3.3. Optimization of Extraction Conditions
3.4. NMR Sample Preparation and Data Acquisition
3.5. NMR Spectrum Processing and Data Preprocessing
3.6. Chemometric Analysis
3.7. Metabolite Identification and Verification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xie, G.Y.; Jiang, Y.X.; Huang, M.M.; Zhu, Y.; Wu, G.; Qin, M.J. Dynamic analysis of secondary metabolites in various parts of Scrophularia ningpoensis by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 186, 113307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ip, F.C.F.; Liang, Y.; Ip, N.Y.; Zhong, B.L.; Lai, C.W.; Xu, S.H. A new iridoid glycoside and a new cinnamoyl glycoside from Scrophularia ningpoensis Hemsl. Nat. Prod. Res. 2017, 31, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.W.; Liu, X.Y.; Cheng, B.; Jia, Z.D.; Hua, H.; Xin, Y. Chemical characterization of polysaccharides isolated from scrophularia ningpoensis and its protective effect on the cerebral ischemia/reperfusin injury in rat model. Int. J. Biol. Macromol. 2019, 139, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Fontaine, J.; Malonne, H.; Claeys, M.; Luhmer, M.; Duez, P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry 2005, 66, 1186–1191. [Google Scholar] [CrossRef]
- Luo, S.B.; Xie, L.P.; Chen, J.J.; Tang, C.R.; Xu, R.A. Determination and pharmacokinetic profiles of four active components from Scrophularia ningpoensis Hemsl. in Rats. Front. Pharmacol. 2021, 11, 612534. [Google Scholar] [CrossRef]
- Gu, W.L.; Chen, C.X.; Wu, Q.; Lu, J.A.; Liu, Y.; Zhang, S.J. Effects of Chinese herb medicine Radix Scrophulariae on ventricular remodeling. Pharmazie 2010, 65, 770–775. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chen, C.X.; Zhang, X.M.; Liu, Y.; Wu, X.M.; Li, Y.M. Effects of ethanolic extract from Radix Scrophulariae on ventricular remodeling in rats. Phytomedicine 2012, 19, 193–205. [Google Scholar] [CrossRef]
- Wang, S.N.; Hua, Y.J.; Zou, L.S.; Liu, X.H.; Yan, Y.; Zhao, H.; Luo, Y.Y.; Liu, J.X. Comparison of chemical constituents in scrophulariae radix processed by different methods based on UFLC-MS combined with multivariate statistical analysis. J. Chromatogr. Sci. 2018, 56, 122–130. [Google Scholar] [CrossRef]
- Li, H.; Liu, P.; Zhang, H.; Feng, W.; Yan, H.; Guo, S.; Qian, D.; Duan, J. Determination of bioactive compounds in the nonmedicinal parts of Scrophularia ningpoensis using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry and chemometric analysis. J. Sep. Sci. 2020, 43, 4191–4201. [Google Scholar] [CrossRef]
- Cao, G.; Cong, X.D.; Cai, H.; Li, X.M.; Ji, J.Y.; Zhang, Y.; Cai, B.C. Simultaneous quantitation of eight active components in crude and processed Radix Scrophulariae extracts by high performance liquid chromatography with diode array detector. Chin. J. Nat. Med. 2012, 10, 213–217. [Google Scholar] [CrossRef]
- Wu, Q.A.; Yuan, Q.A.; Liu, E.H.; Qi, L.W.; Bi, Z.M.; Li, P. Fragmentation study of iridoid glycosides and phenylpropanoid glycosides in Radix Scrophulariae by rapid resolution liquid chromatography with diode-array detection and electrospray ionization time-of-flight mass spectrometry. Biomed. Chromatogr. 2010, 24, 808–819. [Google Scholar] [CrossRef]
- Yang, S.T.; Chen, C.; Zhao, Y.P.; Xi, W.; Zhou, X.L.; Chen, B.L.; Fu, C.X. Association between chemical and genetic variation of wild and cultivated populations of Scrophularia ningpoensis Hemsl. Planta Med. 2011, 77, 865–871. [Google Scholar] [CrossRef]
- Wang, D.H.; Du, F.; Liu, H.Y.; Liang, Z.S. Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J. Med. Plants Res. 2010, 4, 2691–2699. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Zhou, L.; Xu, J.D.; Zhou, S.S.; Shen, H.; Mao, Q.; Kong, M.; Zou, Y.T.; Xu, Y.Y.; Xu, J.; Li, S.L. Chemomics-based marker compounds mining and mimetic processing for exploring chemical mechanisms in traditional processing of herbal medicines, a continuous study on Rehmanniae Radix. J. Chromatogr. A. 2017, 1530, 232–240. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Zhang, L.; Li, B.T.; Feng, Y.L.; Xu, G.L.; Ouyang, H.; Yang, S.L.; Jin, C. Discrimination of toxic ingredient between raw and processed Pinellia ternata by UPLC/Q-TOF-MS/MS with principal component analysis and T-test. Chin. Herb. Med. 2019, 11, 200–208. [Google Scholar] [CrossRef]
- Li, S.L.; Song, J.Z.; Qiao, C.F.; Zhou, Y.; Qian, K.D.; Lee, K.H.; Xu, H.X. A novel strategy to rapidly explore potential chemical markers for the discrimination between raw and processed Radix Rehmanniae by UHPLC-TOFMS with multivariate statistical analysis. J. Pharm. Biomed. Anal. 2010, 51, 812–823. [Google Scholar] [CrossRef]
- Wang, S.N.; Hua, Y.J.; Xu, L.; Zou, L.S.; Liu, X.H.; Luo, Y.Y.; Liu, J.X.; Yan, Y. Quality evaluation of scrophulariae radix processed by different ‘sweating’ methods based on simultaneous determination of multiple bioactive constituents combined with grey relational analysis. Molecules 2016, 21, 850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.K.; Lv, Q.T.; Sun, X.M.; Wang, H.; Li, H.X. On the historical evolution of processing Radix Scrophulariae. Shandong J. Tradit. Chin. Med. 2007, 26, 1–3. [Google Scholar]
- Klare, J.; Rurik, M.; Rottmann, E.; Bollen, A.; Kohlbacher, O.; Fischer, M.; Hackl, T. Determination of the geographical origin of Asparagus officinalis L. by H-1 NMR spectroscopy. J. Agric. Food Chem. 2020, 68, 14353–14363. [Google Scholar] [CrossRef]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Yuk, J.; Simpson, M.J.; Simpson, A.J. 1-D and 2-D NMR-based metabolomics of earthworms exposed to endosulfan and endosulfan sulfate in soil. Environ. Pollut. 2013, 175, 35–44. [Google Scholar] [CrossRef]
- Kim, H.C.; Ko, Y.J.; Jo, C. Potential of 2D qNMR spectroscopy for distinguishing chicken breeds based on the metabolic differences. Food Chem. 2021, 342, 128316. [Google Scholar] [CrossRef]
- Kerem, B. Recent advances in targeted and untargeted metabolomics by NMR and MS/NMR methods. High-Throughput 2018, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Richardson, A.D.; Osterman, A.; Smith, J.W. Profiling of central metabolism in human cancer cells by two-dimensional NMR, GC-MS analysis, and isotopomer modeling. Metabolomics 2008, 4, 13–29. [Google Scholar] [CrossRef]
- Robinette, S.L.; Ajredini, R.; Rasheed, H.; Zeinomar, A.; Schroeder, F.C.; Dossey, A.T.; Edison, A.S. Hierarchical alignment and full resolution pattern recognition of 2D NMR spectra: Application to nematode chemical ecology. Anal. Chem. 2011, 83, 1649–1657. [Google Scholar] [CrossRef]
- Kim, H.C.; Baek, K.H.; Ko, Y.J.; Lee, H.J.; Yim, D.G.; Jo, C. Characteristic metabolic changes of the crust from dry-aged beef using 2D NMR spectroscopy. Molecules 2020, 25, 3087. [Google Scholar] [CrossRef]
- Chae, Y.K.; Kim, S.H.; Nam, Y.K. Application of two-dimensional NMR spectroscopy to metabotyping laboratory Escherichia coli Strains. Chem. Biodivers. 2013, 10, 1816–1827. [Google Scholar] [CrossRef]
- Ai, Z.P.; Zhang, Y.; Li, X.Y.; Sun, W.L.; Liu, Y.H. Widely targeted metabolomics analysis to reveal transformation mechanism of cistanche deserticola active compounds during steaming and drying processes. Front. Nutr. 2021, 8, 742511. [Google Scholar] [CrossRef]
- Jin, J.; Lao, J.; Zhou, R.R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.H.; et al. Simultaneous identification and dynamic analysis of saccharides during steam processing of rhizomes of polygonatum cyrtonema by HPLC-QTOF-MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef] [Green Version]
- Chylla, R.A.; Hu, K.F.; Effinger, J.J.; Markley, J.L. Deconvolution of two-dimensional NMR spectra by fast maximum likelihood reconstruction: Application to quantitative metabolomics. Anal. Chem. 2011, 83, 4871–4880. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, S.Y.; Chun, Y.S.; Chun, Y.J.; Shin, S.Y.; Choi, C.H.; Choi, H.K. Characteristics of fecal metabolic profiles in patients with irritable bowel syndrome with predominant diarrhea investigated using H-1-NMR coupled with multivariate statistical analysis. Neurogastroenterol. Motil. 2020, 32, e13830. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Nguyen, A.T.; Nacoulma, A.P.; Sheridan, H.; Wang, J.F.; Guendouze, N.; Madani, K.; Duez, P. Discrimination of Mentha species grown in different geographical areas of algeria using H-1-NMR-based metabolomics. J. Pharm. Biomed. Anal. 2020, 189, 113430. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shen, C.X.; Pei, X.P.; Li, A.P.; Du, H.Z.; Qin, X.M.; Du, C.H. Compare the chemical profiling of Ziziphi Spinosae Semen and Ziziphi Mauritianae Semen using untargeted metabolomics. J. Food Compos. Anal. 2020, 94, 103635. [Google Scholar] [CrossRef]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by H-1 NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef]
- Masetti, O.; Nisini, L.; Ciampa, A.; Dell’Abate, M.T. H-1 NMR spectroscopy coupled with multivariate analysis was applied to investigate Italian cherry tomatoes metabolic profile. J. Chemometr. 2020, 34, e3191. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Rosas-Bautista, A.; Rico-Arzate, E.; Cruz-Narvaez, Y.; Zepeda-Vallejo, L.G.; Lalaleo, L.; Hidalgo-Martinez, D.; Becerra-Martinez, E. Study of nutritional quality of pomegranate (Punica granatum L.) juice using H-1 NMR-based metabolomic approach: A comparison between conventionally and organically grown fruits. LWT 2020, 134, 110222. [Google Scholar] [CrossRef]
- Huang, T.; Chen, P.; Liu, B.; Li, X.; Lv, X.; Hu, K. NPid: An automatic approach to rapid identification of known natural products in the crude extract of crabapple based on 2D H-1-C-13 heteronuclear correlation spectra of the extract mixture. Anal. Chem. 2020, 92, 10996–11006. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Zhang, M.; Du, H.; Gu, X.; Bai, C.; Zhang, L.; Chen, K.; Hu, K.; Li, Y. Effect of Different Processing Methods on the Chemical Constituents of Scrophulariae Radix as Revealed by 2D NMR-Based Metabolomics. Molecules 2022, 27, 4687. https://doi.org/10.3390/molecules27154687
Duan X, Zhang M, Du H, Gu X, Bai C, Zhang L, Chen K, Hu K, Li Y. Effect of Different Processing Methods on the Chemical Constituents of Scrophulariae Radix as Revealed by 2D NMR-Based Metabolomics. Molecules. 2022; 27(15):4687. https://doi.org/10.3390/molecules27154687
Chicago/Turabian StyleDuan, Xiaohui, Mina Zhang, Huan Du, Xiu Gu, Caihong Bai, Liuqiang Zhang, Kaixian Chen, Kaifeng Hu, and Yiming Li. 2022. "Effect of Different Processing Methods on the Chemical Constituents of Scrophulariae Radix as Revealed by 2D NMR-Based Metabolomics" Molecules 27, no. 15: 4687. https://doi.org/10.3390/molecules27154687