A Cheminformatics Study Regarding the Human Health Risks Assessment of the Stereoisomers of Difenoconazole
Abstract
:1. Introduction
2. Results and Discussions
2.1. ADMET Profiles of the Stereoisomers of Difenoconazole
2.2. Endocrine Disrupting Effects on Humans of the Stereoisomers of Difenoconazole
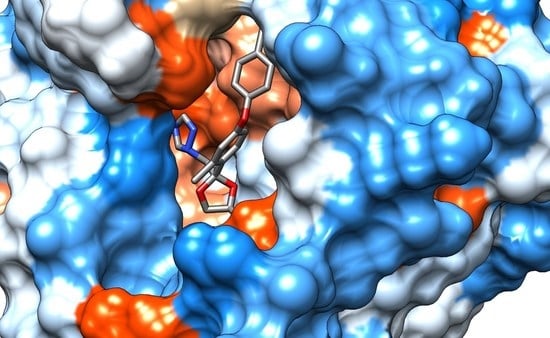
2.3. Assessment of the Interactions of the Stereoisomers of Difenoconazole with Human Cytochromes and Plasma Proteins
2.4. Prediction of Metabolism Sites
3. Materials and Methods
3.1. Stereoisomers of Difenoconazole
3.2. Prediction of the Human Health Hazards of Difenoconazole
3.3. Molecular Docking Study
3.4. Prediction of Metabolism Sites
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caglayan, C.; Taslimi, P.; Türk, C.; Gulcin, İ.; Kandemir, F.M.; Demir, Y.; Beydemir, Ş. Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ. Sci. Pollut. Res. 2020, 10, 10607–10616. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Pan, C.; Jiang, S.; Liu, F. Application of matrix solid-phase dispersion and liquid chromatography–mass spectrometry to fungicide residue analysis in fruits and vegetables. Anal. Bioanal. Chem. 2007, 387, 673–685. [Google Scholar] [CrossRef]
- Khan, N.; Yaqub, G.; Hafeez, T.; Tariq, M. Assessment of health risk due to pesticide residues in fruits, vegetables, soil, and water. J. Chem. 2020, 2020, 5497952. [Google Scholar] [CrossRef]
- Guo, Y. Determination of fungicide residues. Nat. Food 2021, 2, 138. [Google Scholar] [CrossRef]
- de Oliveira Mozzaquatro, J.; Araújo César, I.; Barbosa Pinheiro, A.E.; Caldas, E.D. Pesticide residues analysis in passion fruit and its processed products by LC–MS/MS and GC–MS/MS: Method validation, processing factors and dietary risk assessment. Food Chem. 2022, 375, 131643. [Google Scholar] [CrossRef]
- Lin, S.; Tang, T.; Cang, T.; Yu, S.; Ying, Z.; Gu, S.; Zhang, Q. The distributions of three fungicides in vegetables and their potential health risks in Zhejiang, China: A 3-year study (2015–2017). Environ. Pollut. 2020, 267, 115481. [Google Scholar] [CrossRef]
- Grewal, A.S.; Singla, A.; Kamboj, P.; Dua, J.S. Pesticide residues in food grains, vegetables and fruits: A hazard to human health. J. Med. Chem. Toxicol. 2017, 2, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total. Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Horsfield, A.; Wicks, T.; Davies, K.; Wilson, D.; Paton, S. Effect of fungicide use strategies on the control of early blight (Alternaria solani) and potato yield. Australas. Plant Pathol. 2010, 39, 368–375. [Google Scholar] [CrossRef]
- Jardim, A.N.O.; Caldas, E.D. Brazilian monitoring programs for pesticide residues in food-Results from 2001 to 2010. Food Control. 2012, 25, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Kjaerstad, M.B.; Taxvig, C.; Nellemann, C.; Vinggaard, A.M.; Andersen, H.R. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod. Toxicol. 2010, 30, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Gridan, I.M.; Ciorsac, A.A.; Isvoran, A. Prediction of ADME-Tox properties and toxicological endpoints of triazole fungicides used for cereals protection. ADMET DMPK 2019, 7, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: A review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Taxvig, C.; Hass, U.; Axelstad, M.; Dalgaard, M.; Boberg, J.; Andeasen, H.R.; Vinggaard, A.M. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol. Sci. 2007, 100, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Reigart, J.R.; Roberts, J.R. Recognition and Management of Pesticide Poisoning, 6th ed.; U.S. EPA Office of Pesticide Programs: Washington, DC, USA, 2013. Available online: https://www.epa.gov/pesticide-worker-safety/pesticide-poisoning-handbook-complete-document (accessed on 22 March 2022).
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int. J. Environ. Res. Public Health. 2017, 14, 833. [Google Scholar] [CrossRef] [Green Version]
- Jankuloska, V.; Karov, I.; Pavlovska, G. Residue analysis of difenoconazole in apple fruits grown in Republic of Macedonia. J. Agric. Sci. Technol. 2018, 10, 63–66. [Google Scholar] [CrossRef]
- Akhtar, S.; Yaqub, G.; Hamid, A.; Afzal, Z.; Asghar, S. Determination of pesticide residues in selected vegetables and fruits from a local market of Lahore, Pakistan. Curr. World Environ. 2018, 13, 242–250. [Google Scholar] [CrossRef]
- Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Oriol Magrans, J.; et al. Modification of the existing maximum residue levels fordifenoconazole in leafy brassica. EFSA J. 2021, 19, 6407. [Google Scholar]
- Chen, J.N.; Lian, Y.J.; Zhou, Y.R.; Wang, M.H.; Zhang, X.Q.; Wang, J.H.; Wu, Y.N.; Wang, M.L. Determination of 107 pesticide residues in wolfberry with acetate-buffered salt extraction and Sin-QuEChERS nano column purification coupled with Ultra Performance Liquid Chromatography tandem Mass Spectrometry. Molecules 2019, 24, 2918. [Google Scholar] [CrossRef] [Green Version]
- Elgueta, S.; Valenzuela, M.; Fuentes, M.; Meza, P.; Manzur, J.P.; Liu, S.; Zhao, G.; Correa, A. Pesticide residues and health risk assessment in tomatoes and lettuces from farms of metropolitan region Chile. Molecules 2020, 25, 355. [Google Scholar] [CrossRef] [Green Version]
- EPA-HQ-OPP-2020-0082-0004 Difenoconazole. Human Health Risk Assessment for the Establishment of Tolerances with No U.S. Registrations in/on Japanese Persimmon, Olive, and Black Pepper. Available online: https://www.regulations.gov/document/ (accessed on 20 April 2022).
- Zhuang, S.L.; Bao, L.L.; Wang, H.F.; Zhang, M.; Yang, C.; Zhou, X.Y.; Wu, Y.; Rehman, K.; Naranmandura, H. The involvement of ER-stress and ROS generation in difenoconazole-induced hepatocellular toxicity. Toxicol. Res. 2015, 4, 1195–1203. [Google Scholar] [CrossRef]
- Registration Decision for Difenoconazole. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/cps-spc/alt_formats/pdf/pubs/pest/_decisions/rd2015-15/rd2015-15-eng.pdf (accessed on 20 April 2022).
- Abd-Alrahman, S.H.; Elhalwagy, M.E.; Kotb, G.A.; Farid, H.; Farag, A.A.; Draz, H.M.; Isa, A.M.; Sabico, S. Exposure to difenoconazole, diclofop-methyl alone and combination alters oxidative stress and biochemical parameters in albino rats. Int. J. Clin. Exp. Med. 2014, 7, 3637–3646. [Google Scholar] [PubMed]
- Mu, X.; Pang, S.; Sun, X.; Gao, J.; Chen, J.; Chen, X.; Li, X.; Wang, C. Evaluation of acute and developmental effects of difenoconazole via multiple stage zebrafish assays. Environ. Pollut. 2013, 175, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Qi, S.; Zhu, W.; Wang, Y.; Wang, D.; Yang, Y.; Li, H.; Li, C.; Dong, K.; Wang, C. Sex-specific effects of difenoconazole on the growth hormone endocrine axis in adult zebrafish (Danio rerio). Ecotoxicol. Environ Saf. 2017, 144, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhang, W.; Jia, L.; Li, L.; Zhao, J.; Zhao, Z.; Peng, S.; Chen, Y.; Yuan, X. Combined developmental toxicity of the pesticides difenoconazole and dimethomorph on embryonic zebrafish. Toxins 2021, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, Y.; He, J.; Cheng, H.; Martyniuk, C.J. Endocrine disruption by azole fungicides in fish: A review of the evidence. Sci. Total Environ. 2022, 822, 153412. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral toxicology: It’s the same thing…only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Garrison, A.W.; Avants, J.K.; Jones, W.J. Microbial transformation of triadimefon to triadimenol in soils: Selective production rates of triadimenol stereoisomers affect exposure and risk. Environ. Sci. Technol. 2011, 45, 2186–2193. [Google Scholar] [CrossRef]
- Li, L.; Gao, B.; Wen, Y.; Zhang, Z.; Chen, R.; He, Z.; Kaziem, A.E.; Shi, H.; Wang, M. Stereoselective bioactivity, toxicity and degradation of the chiral triazole fungicide bitertanol. Pest. Manag. Sci. 2020, 76, 343–349. [Google Scholar] [CrossRef]
- Yang, X.; Gong, R.; Chu, Y.; Liu, S.; Xiang, D.; Li, C. Mechanistic insights into stereospecific antifungal activity of chiral fungicide prothioconazole against Fusarium oxysporum F. sp. cubense. Int. J. Mol. Sci. 2022, 23, 2352. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, G.; Bao, Z.; Jin, Y.; Wang, J.; Chen, J.; Qian, M. Stereoselective effects of fungicide difenoconazole and its four stereoisomers on gut barrier, microbiota, and glucolipid metabolism in male mice. Sci. Total Environ. 2022, 805, 150454. [Google Scholar] [CrossRef] [PubMed]
- European Patent Specification, Difenoconazole Stereoisomeric Composition with Reduced Phytotoxicity, EP 2 950 653 B1. Available online: https://data.epo.org/publication-server/document?iDocId=5829367&iFormat=0 (accessed on 25 March 2022).
- Dong, F.; Li, J.; Chankvetadze, B.; Cheng, Y.; Xu, J.; Liu, X.; Li, Y.; Chen, X.; Bertucci, C.; Tedesco, D.; et al. Chiral triazole fungicide difenoconazole: Absolute stereochemistry, stereoselective bioactivity, aquatic toxicity, and environmental behavior in vegetables and soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Isvoran, A.; Ciorsac, A.; Ostafe, V. ADME-Tox profiling of some low molecular weight water soluble chitosan derivatives. ADMET DMPK 2017, 5, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Roman, M.; Roman, D.L.; Ostafe, V.; Ciorsac, A.; Isvoran, A. Computational assessment of pharmacokinetics and biological effects of some anabolic and androgen steroids. Pharm. Res. 2018, 35, 41. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Roman, M.; Som, C.; Schmutz, M.; Hernandez, E.; Wick, P.; Casalini, T.; Perale, G.; Ostafe, V.; Isvoran, A. Computational assessment of the pharmacological profiles of degradation products of chitosan. Front. Bioeng. Biotechnol. 2019, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Isvoran, A.; Filip, M.; Ostafe, V.; Zinn, M. In silico assessment of pharmacological profile of low molecular weight oligo-hydroxyalkanoates. Front. Bioeng. Biotechnol. 2020, 8, 584010. [Google Scholar] [CrossRef]
- Dascalu, D.; Roman, D.; Filip, M.; Ciorsac, A.; Ostafe, V.; Isvoran, A. Solubility and ADMET profiles of short oligomers of lactic acid. ADMET DMPK 2020, 8, 425–436. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational approaches in preclinical studies on drug discovery and development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef]
- Roman, D.L.; Ostafe, V.; Isvoran, A. Computational assessment of chito-oligosaccharides interactions with plasma proteins. Mar. Drugs. 2021, 19, 120. [Google Scholar] [CrossRef]
- Craciun, D.; Dascalu, D.; Isvoran, A. Computational assessment of the ADME-Tox profiles and harmful effects of the most common used phthalates on the human health. Studia UBB Chem. 2019, 64, 71–92. [Google Scholar] [CrossRef]
- Voiculescu, D.I.; Ostafe, V.; Isvoran, A. Computational assessment of the pharmacokinetics and toxicity of the intensive sweeteners. Farmacia 2021, 69, 1032–1041. [Google Scholar] [CrossRef]
- Alves, V.M.; Muratov, E.N.; Zakharov, A.; Muratov, N.N.; Andrade, C.H.; Tropsha, A. Chemical toxicity prediction for major classes of industrial chemicals: Is it possible to develop universal models covering cosmetics, drugs, and pesticides? Food Chem. Toxicol. 2018, 112, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, N.N.; Yao, Z.J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.P.; Cao, D.S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Chen, X.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Gadaleta, D.; Mangiatordi, G.F.; Catto, M.; Carotti, A.; Nicolotti, O. Applicability Domain for QSAR Models: Where Theory Meets Reality. Int. J. Quant. Struct. Prop. Relatsh. 2016, 1, 45–63. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.L.; Souders, C.L.; Pena-Delgado, C.J.; Nguyen, K.T.; Kroyter, N.; El Ahmadie, N.; Aristizabal-Henao, J.J.; Bowden, J.A.; Martyniuk, C.J. Neurotoxicity assessment of triazole fungicides on mitochondrial oxidative respiration and lipids in differentiated human SH-SY5Y neuroblastoma cells. Neurotoxicology 2020, 80, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525. [Google Scholar]
- Kolšek, K.; Mavri, J.; Sollner Dolenc, M.; Gobec, S.; Turk, S. Endocrine Disruptome—An open source prediction tool for assessing endocrine disruption potential through nuclear receptor binding. J. Chem. Inf. Model. 2014, 54, 1254–1267. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, G.; Gao, B.; Hu, K.; Kaziem, A.E.; Li, L.; He, Z.; Shi, H.; Wang, M. Stereoselective endocrine-disrupting effects of the chiral triazole fungicide prothioconazole and its chiral metabolite. Environ. Pollut. 2019, 251, 30–36. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar]
- Rydberg, P.; Gloriam, D.E.; Olsen, L. The SMARTCyp cytochrome P450 metabolism prediction server. Bioinformatics 2010, 26, 2988–2989. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2019, 8, D1388–D1395. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Basheer, A.A. Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality 2018, 30, 402–406. [Google Scholar] [CrossRef] [PubMed]






| Stereoisomer/Rule | Lipinski | Pfizer | GSK |
|---|---|---|---|
| (2R,4S)-difenoconazole | accepted | rejected | rejected |
| (2S,4R)-difenoconazole | accepted | rejected | rejected |
| (2R,4R)-difenoconazole | accepted | rejected | rejected |
| (2S,4S)-difenoconazole | accepted | rejected | rejected |
| Stereoisomer/Online Tool/ Activity | ADMETlab 2.0 | |||||
|---|---|---|---|---|---|---|
| HIA < 30% | P-gp s | P-gp i | BBBP | PPB (%) | CL (mL/min/kg) | |
| (2R,4S)-difenoconazole | 0.003 | 0.001 | 0.013 | 0.021 | 97.54 | 11.720 |
| (2S,4R)-difenoconazole | 0.004 | 0.000 | 0.005 | 0.072 | 97.96 | 11.662 |
| (2R,4R)-difenoconazole | 0.004 | 0.001 | 0.008 | 0.022 | 97.69 | 10.998 |
| (2S,4S)-difenoconazole | 0.003 | 0.000 | 0.009 | 0.064 | 97.62 | 12.327 |
| admetSAR 2.0 | ||||||
| HOBA | P-gp s | P-gp i | BBBP | PPB | ||
| All stereoisomers | 0.729 | −0.699 | −0.660 | 0.972 | 1.000 | |
| Stereoisomer/ -Online Tool/ Activity | ADMETlab 2.0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 | ||||||
| s | i | s | i | s | i | s | i | s | i | |
| (2R,4S)-difenoconazole | 0.493 | 0.921 | 0.255 | 0.922 | 0.413 | 0.912 | 0.268 | 0.710 | 0.885 | 0.945 |
| (2S,4R)-difenoconazole | 0.784 | 0.941 | 0.358 | 0.925 | 0.155 | 0.896 | 0.179 | 0.775 | 0.911 | 0.945 |
| (2R,4R)-difenoconazole | 0.546 | 0.918 | 0.294 | 0.926 | 0.304 | 0.923 | 0.270 | 0.777 | 0.911 | 0.951 |
| (2S,4S)-difenoconazole | 0.735 | 0.938 | 0.322 | 0.924 | 0.195 | 0.881 | 0.164 | 0.696 | 0.888 | 0.934 |
| admetSAR 2.0 | ||||||||||
| CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 | ||||||
| i | i | s | i | s | i | s | i | |||
| all stereoisomers | 0.634 | 0.792 | 1.000 | 0.730 | 0.895 | 0.804 | 0.679 | 0.864 | ||
| Stereoisomer/Online Tool/Toxicological Endpoint | ADMETlab 2.0 | |||||||
|---|---|---|---|---|---|---|---|---|
| hERG | DILI | AMESToxicity | Skin Sensibilization | Carcinogenicity | Eye Corrosion | Eye Irritation | Respiratory Toxicity | |
| (2R,4S)-difenoconazole | 0.729 | 0.985 | 0.960 | 0.903 | 0.671 | 0.003 | 0.682 | 0.020 |
| (2S,4R)-difenoconazole | 0.705 | 0.987 | 0.926 | 0.910 | 0.487 | 0.003 | 0.226 | 0.026 |
| (2R,4R)-difenoconazole | 0.627 | 0.988 | 0.957 | 0.916 | 0.396 | 0.003 | 0.503 | 0.026 |
| (2S,4S)-difenoconazole | 0.807 | 0.982 | 0.944 | 0.900 | 0.736 | 0.003 | 0.266 | 0.020 |
| admetSAR 2.0 | ||||||||
| HT | AMES toxicity | Skin sensibilization | Carcinogenicity | Eye corrosion | Eye irritation | Respiratory toxicity | NT | |
| all stereoisomers | 0.600 | 0.600 | 0.846 | 0.900 | 0.986 | 0.967 | 0.800 | 0.620 |
| Stereoisomer/Nuclear Receptor Inhibition | ADMETlab 2.0 | |||||
|---|---|---|---|---|---|---|
| AR | AR-LB | ER | ER-LB | PPAR γ | ||
| (2R,4S)-difenoconazole | 0.000 | 0.011 | 0.146 | 0.029 | 0.007 | |
| (2S,4R)-difenoconazole | 0.001 | 0.020 | 0.265 | 0.057 | 0.007 | |
| (2R,4R)-difenoconazole | 0.001 | 0.014 | 0.251 | 0.086 | 0.010 | |
| (2S,4S)-difenoconazole | 0.003 | 0.023 | 0.557 | 0.133 | 0.012 | |
| admetSAR 2.0 | ||||||
| All stereoisomers | Reproductive toxicity | AR | ER | TYR | GR | PPAR γ |
| All stereoisomers | 0.878 | 0.869 | 0.932 | 0.806 | 0.897 | 0.764 |
| Stereoisomer/Ligand | Protein and Types of Interactions | ||
|---|---|---|---|
| CYP1A2 | |||
| Hydrophobic | Hydrogen bonds | aromatic donor–acceptor interactions | |
| BFH | THR118, PHE125, PHE226, PHE260(2), ALA317, ASP320, THR321, LEU382, ILE386, LEU497, THR498 | - | PHE226 |
| (2R,4S)-difenoconazole | THR124, VAL227, PHE256, ALA317, ASP320, LEU382, ILE386 | ARG108, ARG137, ARG456 | PHE226 |
| (2S,4R)-difenoconazole | THR124, PHE226, PHE256, PHE260, ALA317, ASP320, THR321, LEU382, ILE386, LEU497 | ILE386 | PHE226 |
| (2R,4R)-difenoconazole | THR124, PHE125 (2), PHE226, ALA317, ASP320, ILE386 | ARG108, THR124, ARG137, ARG456 | - |
| (2S,4S)-difenoconazole | THR124, PHE226 (3), VAL227, ALA317, ASP320, LEU382, ILE386 | ARG108, ARG137, ARG456 | - |
| CYP2C19 | |||
| Hydrophobic | Hydrogen bonds | aromatic donor–acceptor interactions | |
| OXV | VAL113(2), PHE114, ILE205, VAL208, GLU300(2), THR301, ILE362, LEU366, PHE476(2), ALA477 | - | - |
| (2R,4S)-difenoconazole | ALA106, VAL113, VAL208, LEU237, ALA297, THR301, LEU366, | LEU201 | - |
| (2S,4R)-difenoconazole | ILE205, ALA297, THR301, ILE362, LEU366 (2) | ASN204 | - |
| (2R,4R)-difenoconazole | VAL113, PHE114, VAL208, ALA297, THR301, PHE476 (2) | ASN204 | PHE114 |
| (2S,4S)-difenoconazole | ILE205, LEU237, ALA292, ALA297, THR301, PHE476 | - | - |
| CYP2C9 | |||
| Hydrophobic | Hydrogen bonds | aromatic donor–acceptor interactions | |
| SWF | ARG97, PHE100(2), VAL113, LEU208, LEU366(2), PRP367, LEU388, PHE476 | PHE100, ALA103, ASN217 | PHE114, PHE476 |
| (2R,4S)-difenoconazole | ILE205, GLU300, LEU362, ALA477 | GLY98, PHE100 | - |
| (2S,4R)-difenoconazole | PHE100, LEU201, ASN204, ILE205, LEU208 (2), LEU366, PRO367, PHE476 | GLY98 | - |
| (2R,4R)-difenoconazole | LEU102, ALA103, PHE114, LEU208, VAL237 | - | - |
| (2S,4S)-difenoconazole | VAL113, PHE114 (2), VAL237, PHE476 | PHE100 | - |
| CYP2D6 | |||
| Hydrophobic | Hydrogen bonds | aromatic donor–acceptor interactions | |
| Q19 | PHE120(2), LEU121(2), VAL370, VAL374 | - | - |
| (2R,4S)-difenoconazole | This stereoisomer does not bind to the active site of CYP2D6 | ||
| (2S,4R)-difenoconazole | LEU121, THR309, VAL370, VAL374 | PHE120 | - |
| (2R,4R)-difenoconazole | PHE120, LEU121, GLU216, THR309, VAL370, VAL374 (2) | PHE120 | GLU216 |
| (2S,4S)-difenoconazole | PHE120, ALA305, THR309, VAL370, VAL374 | PRO435 | - |
| CYP3A4 | |||
| Hydrophobic | Hydrogen bonds | Salt bridges | |
| MYT | ILE118, PHE137, ILE301(2), PHE302, PHE304, ALA305, THR309, LEU373, PHE435, ILE443 | SER119, SER437 | ARG105 (2), ARG130, ARG375, ARG440 |
| (2R,4S)-difenoconazole | ALA305, THR309, LEU364, ILE369, PHE435, ILE443 | ARG105(2), ARG130, ILE443 | - |
| (2S,4R)-difenoconazole | ILE301, ALA305, THR309, PHE435 | ARG105(2), ARG130, ARG440 | - |
| (2R,4R)-difenoconazole | ILE301, ALA305, ALA370 | ARG105(2), ARG130, ARG440, ILE443 | - |
| (2S,4S)-difenoconazole | ILE301, ALA305, THR309, LEU364, ILE369, PHE435 | ARG105(2), ARG130, ILE443 | - |
| AGP | |||
| Hydrophobic | Hydrogen bonds | aromatic donor–acceptor interactions | |
| JIM | ARG90 | ARG90 | |
| (2R,4S)-difenoconazole | PHE32, TYR37, VAL41, VAL92 (2) | ARG90 | TYR37 |
| (2S,4R)-difenoconazole | TYR27, THR47, VAL92, PHE114 (3) | ARG90 | - |
| (2R,4R)-difenoconazole | PHE32, TYR37, ILE44, THR47, VAL92 (2), PHE114 | ARG90 | - |
| (2S,4S)-difenoconazole | TYR27, PHE32 (2), TYR37, PHE114 (3) | ARG90 | - |
| HSA | |||
| Hydrophobic | Hydrogen bonds | aromatic donor–acceptor interactions/ salt bridges | |
| DIF | ARG218, LEU219, LEU260 | LYS199, ARG218 | |
| (2R,4S)-difenoconazole | LEU219, PHE223, LEU238, ILE264 | ARG257 | salt bridges LYS199, HIS242 |
| (2S,4R)-DFC | ARG218, LEU219, ARG222, LEU238 | LYS199 (2) | - |
| (2R,4R)-difenoconazole | GLN196, LEU219, PHE223, LEU238, VAL241, ILE290 | LYS199 | - |
| (2S,4S)-difenoconazole | TYR150, LEU219, PHE223, ILE260, ILE290 | LYS199 | aromatic donor–acceptor interactions ARG257 |
| Protein/Stereoisomer | ΔG (kcal/mol) | ||||
|---|---|---|---|---|---|
| Ligand in Crystallographic Structure | (2R,4S)-Difenoconazole | (2S,4R)-Difenoconazole | (2R,4R)-Difenoconazole | (2S,4S)-Difenoconazole | |
| CYP1A2 | −8.62 | −9.46 | −10.45 | −9.69 | −9.38 |
| CYP2C19 | −7.47 | −7.64 | −7.77 | −7.64 | −8.83 |
| CYP2C9 | −7.73 | −8.19 | −7.81 | −8.05 | −8.36 |
| CYP2D6 | −8.71 | It does not bind in the active site | −7.56 | −7.34 | −8.06 |
| CYP3A4 | −6.67 | −11.33 | −12.18 | −11.31 | −11.20 |
| HSA | −7.37 | −9.41 | −9.67 | −10.50 | −10.27 |
| AGP | −6.16 | −8.48 | −9.15 | −8.77 | −9.18 |
| All Stereoisomers | Cytochrome | Score/Atoms | ||
| C8 | C3 | C2 | ||
| CYP1A2, CYP3A4 | 49.00 | 53.90 | 54.90 | |
| CYP2C9, CYP2C19 | 62.30 | 72.60 | 73.50 | |
| CYP2D6 | 63.10 | 74.20 | 75.10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voiculescu, D.I.; Roman, D.L.; Ostafe, V.; Isvoran, A. A Cheminformatics Study Regarding the Human Health Risks Assessment of the Stereoisomers of Difenoconazole. Molecules 2022, 27, 4682. https://doi.org/10.3390/molecules27154682
Voiculescu DI, Roman DL, Ostafe V, Isvoran A. A Cheminformatics Study Regarding the Human Health Risks Assessment of the Stereoisomers of Difenoconazole. Molecules. 2022; 27(15):4682. https://doi.org/10.3390/molecules27154682
Chicago/Turabian StyleVoiculescu, Denisa Ioana, Diana Larisa Roman, Vasile Ostafe, and Adriana Isvoran. 2022. "A Cheminformatics Study Regarding the Human Health Risks Assessment of the Stereoisomers of Difenoconazole" Molecules 27, no. 15: 4682. https://doi.org/10.3390/molecules27154682