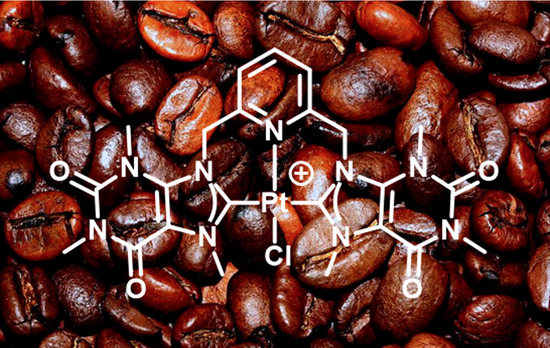
Noble Metal Complexes of a Bis-Caffeine Containing NHC Ligand
Abstract
:1. Introduction
2. Results
2.1. Crystallographic Data
2.2. Photophysical Properties
3. Discussion and Conclusions
4. Materials and Methods
General Procedures
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- Guisado-Barrios, G.; Soleilhavoup, M.; Bertrand, G. 1H-1,2,3-Triazol-5-ylidenes: Readily Available Mesoionic Carbenes. Acc. Chem. Res. 2018, 51, 3236–3244. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M(NHC) (NHC=N-heterocyclic carbene) Bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- Back, O.; Henry-Ellinger, M.; Martin, C.D.; Martin, D.; Bertrand, G. 31P NMR Chemical Shifts of Carbene-Phosphinidene Adducts as an Indicator of the π-Accepting Properties of Carbenes. Angew. Chem. Int. Ed. Engl. 2013, 52, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Santini, C.; Marinelli, M.; Pellei, M. Boron-Centered Scorpionate-Type NHC-Based Ligands and Their Metal Complexes. Eur. J. Inorg. Chem. 2016, 2016, 2312–2331. [Google Scholar] [CrossRef]
- Savka, R.; Plenio, H. Metal Complexes of Very Bulky N,N′-Diarylimidazolylidene N-Heterocyclic Carbene (NHC) Ligands with 2,4,6-Cycloalkyl Substituents. Eur. J. Inorg. Chem. 2014, 2014, 6246–6253. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Persson, P.; Sundstrom, V.; Warnmark, K. Fe N-Heterocyclic Carbene Complexes as Promising Photosensitizers. Acc. Chem. Res. 2016, 49, 1477–1485. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Kjaer, K.S.; Fredin, L.A.; Chabera, P.; Harlang, T.; Canton, S.E.; Lidin, S.; Zhang, J.X.; Lomoth, R.; Bergquist, K.E.; et al. A Heteroleptic Ferrous Complex with Mesoionic Bis(1,2,3-triazol-5-ylidene) Ligands: Taming the MLCT Excited State of Iron(II). Chem. Eur. J. 2015, 21, 3628–3639. [Google Scholar] [CrossRef] [Green Version]
- Schulze, B.; Escudero, D.; Friebe, C.; Siebert, R.; Görls, H.; Köhn, U.; Altuntas, E.; Baumgaertel, A.; Hager, M.D.; Winter, A.; et al. A Heteroleptic Bis(tridentate) Ruthenium(II) Complex of a Click-Derived Abnormal Carbene Pincer Ligand with Potential for Photosensitzer Application. Chem.—A Eur. J. 2011, 17, 5494–5498. [Google Scholar] [CrossRef]
- Tendera, L.; Schaub, T.; Krahfuss, M.J.; Kuntze-Fechner, M.W.; Radius, U. Large vs. Small NHC Ligands in Nickel(0) Complexes: The Coordination of Olefins, Ketones and Aldehydes at [Ni(NHC) 2 ]. Eur. J. Inorg. Chem. 2020, 2020, 3194–3207. [Google Scholar] [CrossRef]
- Liang, Q.; Song, D. Iron N-heterocyclic Carbene Complexes in Homogeneous Catalysis. Chem. Soc. Rev. 2020, 49, 1209–1232. [Google Scholar] [CrossRef]
- Li, J.; He, D.; Lin, Z.; Wu, W.; Jiang, H. Recent Advances in NHC–palladium Catalysis for Alkyne Chemistry: Versatile Synthesis and Applications. Org. Chem. Front. 2021, 8, 3502–3524. [Google Scholar] [CrossRef]
- Chábera, P.; Lindh, L.; Rosemann, N.W.; Prakash, O.; Uhlig, J.; Yartsev, A.; Wärnmark, K.; Sundström, V.; Persson, P. Photofunctionality of Iron(III) N-heterocyclic Carbenes and Related d Transition Metal Complexes. Coord. Chem. Rev. 2021, 426, 213517. [Google Scholar] [CrossRef]
- Stoppa, V.; Scattolin, T.; Bevilacqua, M.; Baron, M.; Graiff, C.; Orian, L.; Biffis, A.; Menegazzo, I.; Roverso, M.; Bogialli, S.; et al. Mononuclear and Dinuclear Gold(I) Complexes with a Caffeine-based Di(N-heterocyclic carbene) Ligand: Synthesis, Reactivity and Structural DFT Analysis. N. J. Chem. 2021, 45, 961–971. [Google Scholar] [CrossRef]
- Saha, S.; Yadav, S.; Reshi, N.U.D.; Dutta, I.; Kunnikuruvan, S.; Bera, J.K. Electronic Asymmetry of an Annelated Pyridyl–Mesoionic Carbene Scaffold: Application in Pd(II)-Catalyzed Wacker-Type Oxidation of Olefins. ACS Catal. 2020, 10, 11385–11393. [Google Scholar] [CrossRef]
- Meng, D.; Li, D.; Ollevier, T. Recyclable Iron(II) Caffeine-derived Ionic Salt Catalyst in the Diels–Alder Reaction of Cyclopentadiene and α,β-unsaturated N-acyl-oxazolidinones in Dimethyl Carbonate. RSC Adv. 2019, 9, 21956–21963. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, B.; Stefan, L.; Pirrotta, M.; Monchaud, D.; Bodio, E.; Richard, P.; Le Gendre, P.; Warmerdam, E.; De Jager, M.H.; Groothuis, G.M.M.; et al. Caffeine-based Gold(I)N-Heterocyclic Carbenes as Possible Anticancer Agents: Synthesis and Biological Properties. Inorg. Chem. 2014, 53, 2296–2303. [Google Scholar] [CrossRef]
- Luo, F.-T.; Lo, H.-K. Short Synthesis of bis-NHC-Pd Catalyst Derived from Caffeine and its Applications to Suzuki, Heck, and Sonogashira Reactions in Aqueous Solution. J. Organomet. Chem. 2011, 696, 1262–1265. [Google Scholar] [CrossRef]
- Hu, J.J.; Bai, S.-Q.; Yeh, H.H.; Young, D.J.; Chi, Y.; Hor, T.S.A. N-Heterocyclic Carbene Pt(II) Complexes from Caffeine: Synthesis, Structures and Photoluminescent Properties. Dalton Trans. 2011, 40, 4402–4406. [Google Scholar] [CrossRef]
- Valdés, H.; Canseco-González, D.; Germán-Acacio, J.M.; Morales-Morales, D. Xanthine based N-heterocyclic carbene (NHC) complexes. J. Organomet. Chem. 2018, 867, 51–54. [Google Scholar] [CrossRef]
- Teng, Q.; Zhao, Y.; Lu, Y.; Liu, Z.; Chen, H.; Yuan, D.; Huynh, H.V.; Meng, Q. Synthesis, Characterization, and Catalytic Study of Caffeine-Derived N-heterocyclic Carbene Palladium Complexes. Organometallics 2022, 41, 161–168. [Google Scholar] [CrossRef]
- Scattolin, T.; Caligiuri, I.; Canovese, L.; Demitri, N.; Gambari, R.; Lampronti, I.; Rizzolio, F.; Santo, C.; Visentin, F. Synthesis of New Allyl Palladium Complexes bearing Purine-based NHC Ligands with Antiproliferative and Proapoptotic Activities on Human Ovarian Cancer Cell Lines. Dalton Trans. 2018, 47, 13616–13630. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; et al. Synthesis from Caffeine of a Mixed N-Heterocyclic Carbene−Silver Acetate Complex Active against Resistant Respiratory Pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Garrison, J.C.; Tessier, C.A.; Youngs, W.J. Synthesis and Structural Characterization of N-Heterocyclic Carbene Complexes of Silver(I) and Rhodium(I) from Caffeine. Organometallics 2004, 23, 1928–1931. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Lake, B.R.M.; Laing, T.; Phillips, R.M.; Willans, C.E. Synthesis and Anticancer Activity of Silver(I)–N-heterocyclic Carbene Complexes Derived from the Natural Xanthine Products Caffeine, Theophylline and Theobromine. Dalton Trans. 2015, 44, 7563–7569. [Google Scholar] [CrossRef] [PubMed]
- Landaeta, V.R.; Rodríguez-Lugo, R.E.; Rodríguez-Arias, E.N.; Coll-Gómez, D.S.; González, T. Studies on the Coordination Chemistry of Methylated Xanthines and their Imidazolium Salts. Part 1: Benzyl Derivatives. Transit. Met. Chem. 2010, 35, 165–175. [Google Scholar] [CrossRef]
- Schütz, J.; Herrmann, W.A. Purine-based Carbenes at Rhodium and Iridium. J. Organomet. Chem. 2004, 689, 2995–2999. [Google Scholar] [CrossRef]
- Francescato, G.; Silva, S.M.d.; Leitão, M.I.P.S.; Gaspar-Cordeiro, A.; Giannopoulos, N.; Gomes, C.S.B.; Pimentel, C.; Petronilho, A. Nickel N-heterocyclic Carbene Complexes based on Xanthines: Synthesis and Antifungal Activity on Candida sp. Appl. Organomet. Chem. 2022, 36, e6687. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic Carbene–silver Complexes: A New Class of Antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Muenzner, J.K.; Abu el Maaty, M.A.; Karge, B.; Schobert, R.; Wölfl, S.; Ott, I. A Multi-target Caffeine Derived rhodium(I) N-heterocyclic Carbene Complex: Evaluation of the Mechanism of Action. Dalton Trans. 2016, 45, 13161–13168. [Google Scholar] [CrossRef] [Green Version]
- Makhloufi, A.; Frank, W.; Ganter, C. Converting Caffeine to Electronically Different N-Heterocyclic Carbenes with a Hypoxanthine Backbone. Organometallics 2012, 31, 7272–7277. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Che, C.-M.; Ott, I. Caffeine derived Platinum(II) N-heterocyclic Carbene Complexes with Multiple Anti-cancer Activities. J. Organomet. Chem. 2015, 782, 37–41. [Google Scholar] [CrossRef]
- Gajare, S.P.; Bansode, P.A.; Patil, P.V.; Patil, A.D.; Pore, D.M.; Sonawane, K.D.; Dhanavade, M.J.; Khot, V.M.; Rashinkar, G.S. Anticancer, Antibacterial and Hyperthermia Studies of a Caffeine-based N-heterocyclic Carbene Silver Complex Anchored on Magnetic Nanoparticles. Chemistryselect 2021, 6, 1958–1968. [Google Scholar] [CrossRef]
- Szadkowska, A.; Staszko, S.; Zaorska, E.; Pawłowski, R. A Theophylline based Copper N-heterocyclic Carbene Complex: Synthesis and Activity Studies in Green Media. RSC Adv. 2016, 6, 44248–44253. [Google Scholar] [CrossRef]
- Eslava-Gonzalez, I.; Valdés, H.; Teresa Ramírez-Apan, M.; Hernandez-Ortega, S.; Rosario Zermeño-Ortega, M.; Avila-Sorrosa, A.; Morales-Morales, D. Synthesis of Theophylline-based Iridium(I) N-heterocyclic Carbene Complexes Including Fluorinated-thiophenolate Ligands. Preliminary Evaluation of their In Vitro Anticancer Activity. Inorg. Chim. Acta 2020, 507, 119588. [Google Scholar] [CrossRef]
- Scattolin, T.; Giust, S.; Bergamini, P.; Caligiuri, I.; Canovese, L.; Demitri, N.; Gambari, R.; Lampronti, I.; Rizzolio, F.; Visentin, F. Palladacyclopentadienyl Complexes Bearing Purine-based N-heterocyclic Carbenes: A New Class of Promising Antiproliferative Agents Against Human Ovarian Cancer. Appl. Organomet. Chem. 2019, 33, e4902. [Google Scholar] [CrossRef]
- Merschel, A.; Rottschäfer, D.; Neumann, B.; Stammler, H.-G.; Ghadwal, R.S. Quantifying the Electronic and Steric Properties of 1,3-Imidazole-Based Mesoionic Carbenes (iMICs). Organometallics 2020, 39, 1719–1729. [Google Scholar] [CrossRef]
- Kjær, K.S.; Kaul, N.; Prakash, O.; Chábera, P.; Rosemann, N.W.; Honarfar, A.; Gordivska, O.; Fredin, L.A.; Bergquist, K.-E.; Häggström, L.; et al. Luminescence and reactivity of a charge-transfer excited iron complex with nanosecond lifetime. Science 2019, 363, 249–253. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Y.; Deng, Q.; Chen, J.; Tu, T. Pd(NHC)-catalyzed alkylsulfonylation of boronic acids: A general and efficient approach for sulfone synthesis. Chemical Communications 2017, 53, 12473–12476. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.; Sun, Z.; Tu, T. Suzuki–Miyaura cross-coupling of bulky anthracenyl carboxylates by using pincer nickel N-heterocyclic carbene complexes: An efficient protocol to access fluorescent anthracene derivatives. Chemical Communications 2013, 49, 11539. [Google Scholar] [CrossRef]
- Tu, T.; Feng, X.; Wang, Z.; Liu, X. A robust hydrophilic pyridine-bridged bis-benzimidazolylidene palladium pincer complex: Synthesis and its catalytic application towards Suzuki–Miyaura couplings in aqueous solvents. Dalton Trans. 2010, 39, 10598. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Bysewski, O.A.; Kupfer, S.; Wächtler, M.; Winter, A.; Schubert, U.S.; Dietzek, B. Excitation Energy-Dependent Branching Dynamics Determines Photostability of Iron(II)–Mesoionic Carbene Complexes. Inorg. Chem. 2021, 60, 9157–9173. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Li, T.; Zhao, S.S.; Li, H.; Su, Z. The Effect of Phenyl Group on the Electronic and Phosphorescent Properties of Cyclometalated Analogues of Platinum(II) Terpyridine Complexes: A Theoretical Study. Theor. Chem. Acc. 2009, 124, 29–36. [Google Scholar] [CrossRef]
- Fleetham, T.; Wang, Z.; Li, J. Efficient Deep Blue Electrophosphorescent Devices based on Platinum(II) Bis(n-methyl-imidazolyl)benzene Chloride. Org. Electron. 2012, 13, 1430–1435. [Google Scholar] [CrossRef]
- Song, C.; Tang, J.; Li, J.; Wang, Z.; Li, P.; Zhang, H. Quantum-Chemical Insights into the Phosphorescence Efficiencies of Blue-Emitting Platinum Complexes with Phenylene-Bridged Pincer Ligands. Inorg. Chem. 2018, 57, 12174–12186. [Google Scholar] [CrossRef]
- Fleetham, T.; Ecton, J.; Wang, Z.; Bakken, N.; Li, J. Single-Doped White Organic Light-Emitting Device with an External Quantum Efficiency Over 20%. Adv. Mater. 2013, 25, 2573–2576. [Google Scholar] [CrossRef]
- Zhang, X.; Wright, A.M.; DeYonker, N.J.; Hollis, T.K.; Hammer, N.I.; Webster, C.E.; Valente, E.J. Synthesis, Air Stability, Photobleaching, and DFT Modeling of Blue Light Emitting Platinum CCC-N-Heterocyclic Carbene Pincer Complexes. Organometallics 2012, 31, 1664–1672. [Google Scholar] [CrossRef]
- Lee, C.-S.; Sabiah, S.; Wang, J.-C.; Hwang, W.-S.; Lin, I.J.B. Water-Induced Changes of Photoluminescence of a Pincer-Type N-Heterocyclic Carbene Platinum(II) Complex. Organometallics 2010, 29, 286–289. [Google Scholar] [CrossRef]
- Darari, M.; Francés-Monerris, A.; Marekha, B.; Doudouh, A.; Wenger, E.; Monari, A.; Haacke, S.; Gros, P.C. Towards Iron(II) Complexes with Octahedral Geometry: Synthesis, Structure and Photophysical Properties. Molecules 2020, 25, 5991. [Google Scholar] [CrossRef]
- Schulze, B.; Schubert, U.S. Beyond Click Chemistry—Supramolecular Interactions of 1,2,3-Triazoles. Chem. Soc. Rev. 2014, 43, 2522. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C NMR Spectroscopic Determination of Ligand Donor Strengths Using N-Heterocyclic Carbene Complexes of Palladium(II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Baker, M.V.; Brown, D.H.; Haque, R.A.; Skelton, B.W.; White, A.H. Silver(I) and Mercury(II) Complexes of Meta- and Para-xylyl linked Bis(imidazol-2-ylidenes). J. Incl. Phenom. Macrocycl. Chem. 2009, 65, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Guo, Z.; Ludlow Iii, J.M.; Xie, T.; Liao, S.; Newkome, G.R.; Wesdemiotis, C. Characterization of Metallosupramolecular Polymers by Top-down Multidimensional Mass Spectrometry Methods. Macromol. Rapid Commun. 2015, 36, 1539–1552. [Google Scholar] [CrossRef]
- Serra, D.; Cao, P.; Cabrera, J.; Padilla, R.; Rominger, F.; Limbach, M. Development of Platinum(II) and -(IV) CNC Pincer Complexes and their Application in a Hydrovinylation Reaction. Organometallics 2011, 30, 1885–1895. [Google Scholar] [CrossRef]
- Gründemann, S.; Albrecht, M.; Loch, J.A.; Faller, J.W.; Crabtree, R.H. Tridentate Carbene CCC and CNC Pincer Palladium(II) Complexes: Structure, Fluxionality, and Catalytic Activity. Organometallics 2001, 20, 5485–5488. [Google Scholar] [CrossRef]
- Tulloch, A.A.D.; Danopoulos, A.A.; Tizzard, G.J.; Coles, S.J.; Hursthouse, M.B.; Hay-Motherwell, R.S.; Motherwell, W.B. Chiral 2,6-Lutidinyl-biscarbene Complexes of Palladium. Chem. Commun. 2001, 1270–1271. [Google Scholar] [CrossRef]
- Hahn, F.E.; Jahnke, M.C.; Gomez-Benitez, V.; Morales-Morales, D.; Pape, T. Synthesis and Catalytic Activity of Pincer-Type Bis(benzimidazolin-2-ylidene) Palladium Complexes. Organometallics 2005, 24, 6458–6463. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Gründemann, S.; Albrecht, M.; Mégret, C.; Clot, E.; Faller, J.W.; Eisenstein, O.; Crabtree, R.H. Outer Sphere Anion Participation can Modify the Mechanism for Conformer Interconversion in Pd Pincer Complexes. Dalton Trans. 2003, 5, 831–838. [Google Scholar] [CrossRef]
- Nielsen, D.J.; Cavell, K.J.; Skelton, B.W.; White, A.H. A Pyridine Bridged dicarbene Ligand and its Silver(I) and Palladium(II) Complexes: Synthesis, Structures, and Catalytic Applications. Inorg. Chim. Acta 2002, 327, 116–125. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Tulloch, A.A.D.; Winston, S.; Eastham, G.; Hursthouse, M.B. Chelating and ‘Pincer’ Dicarbene Complexes of Palladium; Synthesis and Structural Studies. Dalton Trans. 2003, 3, 1009–1015. [Google Scholar] [CrossRef]
- Dinda, J.; Adhikary, S.D.; Roymahapatra, G.; Nakka, K.K.; Santra, M.K. Synthesis, Structure, Electrochemistry and Cytotoxicity Studies of Ru(II) and Pt(II)–N-heterocyclic Carbene Complexes of CNC-pincer lLgand. Inorg. Chim. Acta 2014, 413, 23–31. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Dias, H.V.R.; Calabrese, J.C.; Davidson, F. Homoleptic Carbene-silver(I) and Carbene-copper(I) Complexes. Organometallics 1993, 12, 3405–3409. [Google Scholar] [CrossRef]
- Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Group 11 Metal Complexes of N-Heterocyclic Carbene Ligands: Nature of the MetalCarbene Bond. Organometallics 2004, 23, 755–764. [Google Scholar] [CrossRef]
- Che, C.M.; He, L.Y.; Poon, C.K.; Mak, T.C.W. Solid-state Emission of Dicyanoplatinum(II) and -Palladium(II) Complexes of Substituted 2,2′-Bipyridines and 1,10-Phenanthroline and X-ray Crystal Structures of Isomorphous M(bpy)(CN)2 (bpy = 2,2′-bipyridine; M = Pt, Pd). Inorg. Chem. 1989, 28, 3081–3083. [Google Scholar] [CrossRef]
- Abe, T.; Itakura, T.; Ikeda, N.; Shinozaki, K. Luminescence Color Change of a Platinum(II) Complex Solid upon Mechanical Grinding. Dalton Trans. 2009, 38, 711–715. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Wong, K.M.-C.; Zhu, N. Solvent-induced Aggregation through Metal···Metal/π···π Interactions: Large Solvatochromism of Luminescent Organoplatinum(II) Terpyridyl Complexes. J. Am. Chem. Soc. 2002, 124, 6506–6507. [Google Scholar] [CrossRef]
- Ohno, K.; Tanuma, H.; Kusano, Y.; Kaizaki, S.; Nagasawa, A.; Fujihara, T. Luminescence of Tartrate Bridged Dinuclear 2,2--Bipyridine Platinum(ii) Complexes: Emission Color Controlled by Intra- and Inter-molecular Interactions in the Solid State. Dalton Trans. 2017, 46, 7612–7618. [Google Scholar] [CrossRef]
- Connick, W.B.; Marsh, R.E.; Schaefer, W.P.; Gray, H.B. Linear-Chain Structures of Platinum(II) Diimine Complexes. Inorg. Chem. 1997, 36, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Kletsch, L.; Jordan, R.; Köcher, A.S.; Buss, S.; Strassert, C.A.; Klein, A. Photoluminescence of Ni(II), Pd(II), and Pt(II) Complexes [M(Me2dpb)Cl] Obtained from C–H Activation of 1,5-Di(2-pyridyl)-2,4-dimethylbenzene (Me2dpbH). Molecules 2021, 26, 5051. [Google Scholar] [CrossRef]
- Fletcher, A.N. Fluorescence Emission Band Shift with Wavelength of Excitation. J. Phys. Chem. 1968, 72, 2742–2749. [Google Scholar] [CrossRef]
- Panthi, K.; Adhikari, R.M.; Kinstle, T.H. Aromatic Fumaronitrile Core-based Donor−Linker−Acceptor−Linker−Donor (D-π-A-π-D) Compounds: Synthesis and Photophysical Properties. J. Phys. Chem. A 2010, 114, 4542–4549. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.S.; Seliskar, C.J.; McGlynn, S.P. Electronic Spectroscopy of Highly-polar Aromatics.* II. Luminescence of Nitroanilines. J. Chem. Phys. 1973, 58, 1607–1612. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Keating-Nakamoto, S. Red-edge Excitation of Fluorescence and Dynamic Properties of Proteins and Membranes. Biochemistry 1984, 23, 3013–3021. [Google Scholar] [CrossRef]
- Harlepp, S.; Chardon, E.; Bouché, M.; Dahm, G.; Maaloum, M.; Bellemin-Laponnaz, S. N-Heterocyclic Carbene-Platinum Complexes Featuring an Anthracenyl Moiety: Anti-Cancer Activity and DNA Interaction. Int. J. Mol. Sci. 2019, 20, 4198. [Google Scholar] [CrossRef] [Green Version]
- Yersin, H.; Donges, D. Low-Lying Electronic States and Photophysical Properties of Organometallic Pd(II) and Pt(II) Compounds. Modern Research Trends Presented in Detailed Case Studies. In Transition Metal and Rare Earth Compounds: Excited States, Transitions, Interactions II; Yersin, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 81–186. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-group and Crystal-structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]










| Compound | Absorption/nm | Emission/nm |
|---|---|---|
| 4 | 267 | 321.5 a, 400.5 b |
| 6 | 268, 316 | 435.5 a, 420 b |
| 7 | 269, 321 | 441 a, 443.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bysewski, O.; Winter, A.; Liebing, P.; Schubert, U.S. Noble Metal Complexes of a Bis-Caffeine Containing NHC Ligand. Molecules 2022, 27, 4316. https://doi.org/10.3390/molecules27134316
Bysewski O, Winter A, Liebing P, Schubert US. Noble Metal Complexes of a Bis-Caffeine Containing NHC Ligand. Molecules. 2022; 27(13):4316. https://doi.org/10.3390/molecules27134316
Chicago/Turabian StyleBysewski, Oliver, Andreas Winter, Phil Liebing, and Ulrich S. Schubert. 2022. "Noble Metal Complexes of a Bis-Caffeine Containing NHC Ligand" Molecules 27, no. 13: 4316. https://doi.org/10.3390/molecules27134316