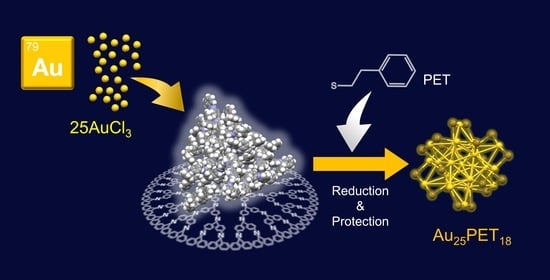
Controlled Synthesis of Au25 Superatom Using a Dendrimer Template
Abstract
:1. Introduction
2. Result and Discussion
2.1. Controlled Assembly of AuCl3 on TPM G4
2.2. Comparison with Existing Methods
2.3. Synthesis of MAu24PET18 Using the Dendrimer TPM G4 as a Template
3. Materials and Methods
3.1. Chemicals
3.2. Assembly Accumulation of AuCl3 on Dendrimer TPM G4
3.3. Synthesis of Au25PET18 Using Dendrimers
3.4. Synthesis of MAu24PET18 (M = Pd, Pt) Using Dendrimers
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsukamoto, T.; Haruta, N.; Kambe, T.; Kuzume, A.; Yamamoto, K. Periodicity of Molecular Clusters Based on Symmetry-Adapted Orbital Model. Nat. Commun. 2019, 10, 3727. [Google Scholar] [CrossRef] [PubMed]
- Ekardt, W. Dynamical polarizability of small metal particles: Self-consistent spherical jellium background model. Phys. Rev. Lett. 1984, 52, 1925–1928. [Google Scholar] [CrossRef]
- Leuchtner, R.E.; Harms, A.C.; Castleman, A.W. Thermal metal cluster anion reactions: Behavior of aluminum clusters with oxygen. J. Chem. Phys. 1989, 91, 2753–2754. [Google Scholar] [CrossRef]
- Luo, Z.; Berkdemir, C.; Smith, J.C.; Castleman, A.W. Cluster reaction of [Ag8]−/[Cu8]− with chlorine: Evidence for the harpoon mechanism? Chem. Phys. Lett. 2013, 582, 24–30. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Chaki, N.K.; Negishi, Y.; Tsunoyama, H.; Shichibu, Y.; Tsukuda, T. Ubiquitous 8 and 29 KDa gold: Alkanethiolate cluster compounds: Mass-spectrometric determination of molecular formulas and structural implications. J. Am. Chem. Soc. 2008, 130, 8608–8610. [Google Scholar] [CrossRef]
- Kimura, K.; Sugimoto, N.; Sato, S.; Yao, H.; Negishi, Y.; Tsukuda, T. Size determination of gold clusters by polyacrylamide gel electrophoresis in a large cluster region. J. Phys. Chem. C 2009, 113, 14076–14082. [Google Scholar] [CrossRef]
- Xie, S.; Tsunoyama, H.; Kurashige, W.; Negishi, Y.; Tsukuda, T. Enhancement in aerobic alcohol oxidation catalysis of Au25 clusters by single Pd atom doping. ACS Catal. 2012, 2, 1519–1523. [Google Scholar] [CrossRef]
- Negishi, Y.; Chaki, N.K.; Shichibu, Y.; Whetten, R.L.; Tsukuda, T. Origin of magic stability of thiolated gold clusters: A case study on Au25(SC6H13)18. J. Am. Chem. Soc. 2007, 129, 11322–11323. [Google Scholar] [CrossRef]
- Negishi, Y.; Takasugi, Y.; Sato, S.; Yao, H.; Kimura, K.; Tsukuda, T. Magic-numbered Aun clusters protected by glutathione monolayers (n = 18, 21, 25, 28, 32, 39): Isolation and spectroscopic characterization. J. Am. Chem. Soc. 2004, 126, 6518–6519. [Google Scholar] [CrossRef]
- Negishi, Y.; Takasugi, Y.; Sato, S.; Yao, H.; Kimura, K.; Tsukuda, T. Kinetic stabilization of growing gold clusters by passivation with thiolates. J. Phys. Chem. B 2006, 110, 12218–12221. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, S.; Kurashige, W.; Nobusada, K.; Negishi, Y.; Tsukuda, T. Preferential location of coinage metal dopants (M = Ag or Cu) in [Au25–xMx(SC2H4Ph)18]−( x ∼ 1) as determined by extended X-ray absorption fine structure and density functional theory calculations. J. Phys. Chem. C 2014, 118, 25284–25290. [Google Scholar] [CrossRef]
- Negishi, Y.; Nakazaki, T.; Malola, S.; Takano, S.; Niihori, Y.; Kurashige, W.; Yamazoe, S.; Tsukuda, T.; Häkkinen, H. A critical size for emergence of nonbulk electronic and geometric structures in dodecanethiolate-protected Au clusters. J. Am. Chem. Soc. 2015, 137, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yamazoe, S.; Ono, T.; Kurashige, W.; Niihori, Y.; Nobusada, K.; Tsukuda, T.; Negishi, Y. Tuning the electronic structure of thiolate-protected 25-atom clusters by co-substitution with metals having different preferential sites. Dalton Trans. 2016, 45, 18064–18068. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Yamashita, K.; Muramatsu, S.; Takano, S.; Ohshimo, K.; Azuma, T.; Nakanishi, R.; Nagata, T.; Yamazoe, S.; Koyasu, K.; et al. Anion photoelectron spectroscopy of free [Au25(SC12H25)18]−. Nanoscale 2017, 9, 13409–13412. [Google Scholar] [CrossRef] [PubMed]
- Omoda, T.; Takano, S.; Tsukuda, T. Toward controlling the electronic structures of chemically modified superatoms of gold and silver. Small 2021, 17, 2001439. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kim, K.; Nakamura, K.; Kitazawa, H.; Hayashi, S.; Koyasu, K.; Tsukuda, T. Photoinduced thermionic emission from [M25(SR)18]− (M = Au, Ag) revealed by anion photoelectron spectroscopy. J. Phys. Chem. C 2019, 123, 13174–13179. [Google Scholar] [CrossRef]
- Omoda, T.; Takano, S.; Tsukuda, T. Reduction-resistant [Au25(Cyclohexanethiolate)18]0 with an icosahedral Au13 core. Chem. Lett. 2019, 48, 885–887. [Google Scholar] [CrossRef] [Green Version]
- Suyama, M.; Takano, S.; Nakamura, T.; Tsukuda, T. Stoichiometric formation of open-shell [PtAu 24 (SC 2 H 4 Ph) 18 ]− via spontaneous electron proportionation between [PtAu24(SC2H4Ph)18]2– and [PtAu24(SC2H4Ph)18]0. J. Am. Chem. Soc. 2019, 141, 14048–14051. [Google Scholar] [CrossRef]
- Suyama, M.; Takano, S.; Tsukuda, T. Synergistic effects of Pt and Cd Codoping to icosahedral Au13 superatoms. J. Phys. Chem. C 2020, 124, 23923–23929. [Google Scholar] [CrossRef]
- Negishi, Y.; Kurashige, W.; Niihori, Y.; Nobusada, K. Toward the creation of stable, functionalized metal clusters. Phys. Chem. Chem. Phys. 2013, 15, 18736. [Google Scholar] [CrossRef] [PubMed]
- Niihori, Y.; Koyama, Y.; Watanabe, S.; Hashimoto, S.; Hossain, S.; Nair, L.V.; Kumar, B.; Kurashige, W.; Negishi, Y. Atomic and isomeric separation of thiolate-protected alloy clusters. J. Phys. Chem. Lett. 2018, 9, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Niihori, Y.; Nair, L.V.; Kumar, B.; Kurashige, W.; Negishi, Y. Alloy clusters: Precise synthesis and mixing effects. Acc. Chem. Res. 2018, 51, 3114–3124. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kurashige, W.; Nobusada, K.; Negishi, Y. Effect of trimetallization in thiolate-protected Au24−nCunPd clusters. Nanoscale 2015, 7, 10606–10612. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Kurashige, W.; Niihori, Y.; Iwasa, T.; Nobusada, K. Isolation, structure, and stability of a dodecanethiolate-protected Pd1Au24 cluster. Phys. Chem. Chem. Phys. 2010, 12, 6219. [Google Scholar] [CrossRef]
- Hossain, S.; Suzuki, D.; Iwasa, T.; Kaneko, R.; Suzuki, T.; Miyajima, S.; Iwamatsu, Y.; Pollitt, S.; Kawawaki, T.; Barrabés, N.; et al. Determining and controlling Cu-substitution sites in thiolate-protected gold-based 25-atom alloy nanoclusters. J. Phys. Chem. C 2020, 124, 22304–22313. [Google Scholar] [CrossRef]
- Negishi, Y.; Iwai, T.; Ide, M. Continuous modulation of electronic structure of stable thiolate-protected Au25 cluster by Ag doping. Chem. Commun. 2010, 46, 4713. [Google Scholar] [CrossRef]
- Negishi, Y.; Munakata, K.; Ohgake, W.; Nobusada, K. Effect of copper doping on electronic structure, geometric structure, and stability of thiolate-protected Au25 nanoclusters. J. Phys. Chem. Lett. 2012, 3, 2209–2214. [Google Scholar] [CrossRef]
- Ohta, T.; Shibuta, M.; Tsunoyama, H.; Negishi, Y.; Eguchi, T.; Nakajima, A. Size and structure dependence of electronic states in thiolate-protected gold nanoclusters of Au25(SR)18, Au38(SR)24, and Au144(SR)60. J. Phys. Chem. C 2013, 117, 3674–3679. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Heaven, M.W.; Dass, A.; White, P.S.; Holt, K.M.; Murray, R.W. Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18]. J. Am. Chem. Soc. 2008, 130, 3754–3755. [Google Scholar] [CrossRef] [PubMed]
- Dolamic, I.; Knoppe, S.; Dass, A.; Bürgi, T. First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. Nat. Commun. 2012, 3, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, H.; Eckenhoff, W.T.; Zhu, Y.; Pintauer, T.; Jin, R. Total structure determination of thiolate-protected Au38 nanoparticles. J. Am. Chem. Soc. 2010, 132, 8280–8281. [Google Scholar] [CrossRef] [PubMed]
- Bahena, D.; Bhattarai, N.; Santiago, U.; Tlahuice, A.; Ponce, A.; Bach, S.B.H.; Yoon, B.; Whetten, R.L.; Landman, U.; Jose-Yacaman, M. STEM electron diffraction and high-resolution images used in the determination of the crystal structure of the Au144(SR)60 cluster. J. Phys. Chem. Lett. 2013, 4, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malola, S.; Lehtovaara, L.; Enkovaara, J.; Häkkinen, H. Birth of the localized surface plasmon resonance in monolayer-protected gold nanoclusters. ACS Nano 2013, 7, 10263–10270. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Jiang, D.; Li, G.; Gayathri, C.; Das, A.; Gil, R.R.; Jin, R. Monoplatinum doping of gold nanoclusters and catalytic application. J. Am. Chem. Soc. 2012, 134, 16159–16162. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.L.; MacDonald, M.A.; Chatt, A.; Zhang, P.; Qian, H.; Jin, R. Dopant location, local structure, and electronic properties of Au24Pt(SR)18 nanoclusters. J. Phys. Chem. C 2012, 116, 26932–26937. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Jin, S.; Liu, X.; Zhang, J.; Pei, Y.; Meng, X.; Chen, M.; Li, P.; Zhu, M. Metal exchange method using Au25 nanoclusters as templates for alloy nanoclusters with atomic precision. J. Am. Chem. Soc. 2015, 137, 4018–4021. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Jin, S.; Chen, S.; Sheng, H.; Zhu, M. A metal exchange method for thiolate-protected tri-metal M1AgxAu24−x(SR)180 (M = Cd/Hg) nanoclusters. Nanoscale 2015, 7, 10005–10007. [Google Scholar] [CrossRef]
- Zhu, M.; Lanni, E.; Garg, N.; Bier, M.E.; Jin, R. Kinetically controlled, high-yield synthesis of Au 25 clusters. J. Am. Chem. Soc. 2008, 130, 1138–1139. [Google Scholar] [CrossRef]
- Dharmaratne, A.C.; Krick, T.; Dass, A. Nanocluster size evolution studied by mass spectrometry in room temperature Au25(SR)18 synthesis. J. Am. Chem. Soc. 2009, 131, 13604–13605. [Google Scholar] [CrossRef] [PubMed]
- Esumi, K.; Miyamoto, K.; Yoshimura, T. Comparison of PAMAM–Au and PPI–Au nanocomposites and their catalytic activity for reduction of 4-nitrophenol. J. Colloid Interface Sci. 2002, 254, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Crooks, R.M. Electrocatalytic O2 reduction at glassy carbon electrodes modified with dendrimer-encapsulated Pt nanoparticles. J. Am. Chem. Soc. 2005, 127, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, C.; Aranzaes, J.R.; Salmon, L.; Astruc, D. “Click” dendrimers: Synthesis, redox sensing of Pd(OAc)2, and remarkable catalytic hydrogenation activity of precise Pd nanoparticles stabilized by 1,2,3-triazole-containing dendrimers. Chem. Eur. J. 2008, 14, 50–64. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.C.; Crooks, R.M. Extraction of Au nanoparticles having narrow size distributions from within dendrimer templates. J. Am. Chem. Soc. 2004, 126, 16170–16178. [Google Scholar] [CrossRef]
- Kéki, S.; Török, J.; Deák, G.; Daróczi, L.; Zsuga, M. Silver nanoparticles by PAMAM-assisted photochemical reduction of Ag+. J. Colloid Interface Sci. 2000, 229, 550–553. [Google Scholar] [CrossRef]
- Ostojic, N.; Duan, Z.; Galyamova, A.; Henkelman, G.; Crooks, R.M. Electrocatalytic study of the oxygen reduction reaction at gold nanoparticles in the absence and presence of interactions with SnOx supports. J. Am. Chem. Soc. 2018, 140, 13775–13785. [Google Scholar] [CrossRef]
- Hayakawa, K.; Yoshimura, T.; Esumi, K. Preparation of gold−dendrimer nanocomposites by laser irradiation and their catalytic reduction of 4-nitrophenol. Langmuir 2003, 19, 5517–5521. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Aihara, N.; Usui, K.; Torigoe, K. Preparation of gold colloids with UV irradiation using dendrimers as stabilizer. Langmuir 1998, 14, 3157–3159. [Google Scholar] [CrossRef]
- Boisselier, E.; Diallo, A.K.; Salmon, L.; Ornelas, C.; Ruiz, J.; Astruc, D. Encapsulation and stabilization of gold nanoparticles with “click” polyethyleneglycol dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar] [CrossRef]
- Ispasoiu, R.G.; Balogh, L.; Varnavski, O.P.; Tomalia, D.A. Goodson large optical limiting from novel metal–dendrimer nanocomposite materials. J. Am. Chem. Soc. 2000, 122, 11005–11006. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Yamahira, A.; Torigoe, K. Role of poly(amidoamine) dendrimers for preparing nanoparticles of gold, platinum, and silver. Langmuir 2000, 16, 2604–2608. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Montalti, F.; Turro, N.J.; Tomalia, D.A. Characterization of starburst dendrimers by the EPR technique. Copper(II) ions binding full-generation dendrimers. J. Phys. Chem. B 1997, 101, 158–166. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Bossmann, S.; Turro, N.J.; Tomalia, D.A. Characterization of starburst dendrimers by the EPR technique. 1. Copper complexes in water solution. J. Am. Chem. Soc. 1994, 116, 661–671. [Google Scholar] [CrossRef]
- He, J.-A.; Valluzzi, R.; Yang, K.; Dolukhanyan, T.; Sung, C.; Kumar, J.; Tripathy, S.K.; Samuelson, L.; Balogh, L.; Tomalia, D.A. Electrostatic multilayer deposition of a gold−dendrimer nanocomposite. Chem. Mater. 1999, 11, 3268–3274. [Google Scholar] [CrossRef]
- Balogh, L.; Valluzzi, R.; Laverdure, K.S.; Gido, S.P.; Hagnauer, G.L.; Tomalia, D.A. Formation of silver and gold dendrimer nanocomposites. J. Nanopart. Res. 1999, 1, 353–368. [Google Scholar] [CrossRef]
- Balogh, L.; Tomalia, D.A. Poly(amidoamine) dendrimer-templated nanocomposites. 1. Synthesis of zerovalent copper nanoclusters. J. Am. Chem. Soc. 1998, 120, 7355–7356. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Enoki, O.; Katoh, H.; Yamamoto, K. Synthesis and properties of a novel phenylazomethine dendrimer with a tetraphenylmethane core. Org. Lett. 2006, 8, 569–571. [Google Scholar] [CrossRef]
- Yamamoto, K.; Higuchi, M.; Shiki, S.; Tsuruta, M.; Chiba, H. Stepwise radial complexation of imine groups in phenylazomethine dendrimers. Nature 2002, 415, 509–511. [Google Scholar] [CrossRef]
- Takanashi, K.; Fujii, A.; Nakajima, R.; Chiba, H.; Higuchi, M.; Einaga, Y.; Yamamoto, K. Heterometal assembly in dendritic polyphenylazomethines. Bull. Chem. Soc. Jpn. 2007, 80, 1563–1572. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imaoka, T.; Chun, W.-J.; Enoki, O.; Katoh, H.; Takenaga, M.; Sonoi, A. Size-specific catalytic activity of platinum clusters enhances oxygen reduction reactions. Nat. Chem. 2009, 1, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Imaoka, S.; Imaoka, T.; Yamamoto, K. Build-up enhancement of photoluminescence from phenylazomethine bismuth dendrimer using Bi(OTf)3. J. Nanopart. Res. 2018, 20, 118. [Google Scholar] [CrossRef]
- Kambe, T.; Watanabe, A.; Imaoka, T.; Yamamoto, K. Bismuth complexes in phenylazomethine dendrimers: Controllable luminescence and emission in the solid state. Angew. Chem. Int. Ed. 2016, 55, 13151–13154. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, T.; Fushimi, M.; Kimoto, A.; Okamoto, Y.; Takanashi, K.; Yamamoto, K. Resistive switching memory based on size-controlled 1-nm gold particles. Chem. Lett. 2014, 43, 1269–1271. [Google Scholar] [CrossRef]
- Takahashi, M.; Koizumi, H.; Chun, W.-J.; Kori, M.; Imaoka, T.; Yamamoto, K. Finely controlled multimetallic nanocluster catalysts for solvent-free aerobic oxidation of hydrocarbons. Sci. Adv. 2017, 3, e1700101. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, T.; Kambe, T.; Nakao, A.; Imaoka, T.; Yamamoto, K. Atom-hybridization for synthesis of polymetallic clusters. Nat. Commun. 2018, 9, 3873. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Li, M.; Tsukamoto, T.; Imaoka, T.; Yamamoto, K. Expansion of Dendrimer Template Function for Subnanoparticle Synthesis. Chem. Lett. 2021, 50, 1648–1651. [Google Scholar] [CrossRef]
- Kambe, T.; Yamamoto, K. Functionalization of Phenylazomethine Dendrimers. Polym J 2022, 54, 97–105. [Google Scholar] [CrossRef]
- Imaoka, T.; Kitazawa, H.; Chun, W.; Yamamoto, K. Finding the most catalytically active platinum clusters with low atomicity. Angew. Chem. Int. Ed. 2015, 54, 9810–9815. [Google Scholar] [CrossRef]
- Kambe, T.; Haruta, N.; Imaoka, T.; Yamamoto, K. Solution-phase synthesis of Al13− using a dendrimer template. Nat. Commun. 2017, 8, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, T.; Watanabe, A.; Li, M.; Tsukamoto, T.; Imaoka, T.; Yamamoto, K. Superatomic gallium clusters in dendrimers: Unique Rigidity and reactivity depending on their atomicity. Adv. Mater. 2020, 32, 1907167. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.; Stevenson, A.; Dubay, G.R.; Tracy, J.B.; Murray, R.W. Nanoparticle MALDI-TOF mass spectrometry without fragmentation: Au25(SCH2CH2Ph)18 and mixed monolayer Au25(SCH2CH2Ph)18−x(L)x. J. Am. Chem. Soc. 2008, 130, 5940–5946. [Google Scholar] [CrossRef] [PubMed]
- Patiny, L.; Borel, A. ChemCalc: A building block for tomorrow’s chemical infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shichibu, Y.; Negishi, Y.; Tsukuda, T.; Teranishi, T. Large-scale synthesis of thiolated Au25 clusters via ligand exchange reactions of phosphine-stabilized Au11 clusters. J. Am. Chem. Soc. 2005, 127, 13464–13465. [Google Scholar] [CrossRef]
- Tracy, J.B.; Kalyuzhny, G.; Crowe, M.C.; Balasubramanian, R.; Choi, J.-P.; Murray, R.W. Poly(ethylene glycol) ligands for high-resolution nanoparticle mass spectrometry. J. Am. Chem. Soc. 2007, 129, 6706–6707. [Google Scholar] [CrossRef]
- Donkers, R.L.; Lee, D.; Murray, R.W. Synthesis and isolation of the molecule-like cluster Au38(PhCH2CH2S)24. Langmuir 2004, 20, 1945–1952. [Google Scholar] [CrossRef]
- Tofanelli, M.A.; Ni, T.W.; Phillips, B.D.; Ackerson, C.J. Crystal structure of the PdAu24(SR)180 superatom. Inorg. Chem. 2016, 55, 999–1001. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muramatsu, H.; Kambe, T.; Tsukamoto, T.; Imaoka, T.; Yamamoto, K. Controlled Synthesis of Au25 Superatom Using a Dendrimer Template. Molecules 2022, 27, 3398. https://doi.org/10.3390/molecules27113398
Muramatsu H, Kambe T, Tsukamoto T, Imaoka T, Yamamoto K. Controlled Synthesis of Au25 Superatom Using a Dendrimer Template. Molecules. 2022; 27(11):3398. https://doi.org/10.3390/molecules27113398
Chicago/Turabian StyleMuramatsu, Hisanori, Tetsuya Kambe, Takamasa Tsukamoto, Takane Imaoka, and Kimihisa Yamamoto. 2022. "Controlled Synthesis of Au25 Superatom Using a Dendrimer Template" Molecules 27, no. 11: 3398. https://doi.org/10.3390/molecules27113398