In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna aconitifolia (Jacq.) Marechal) Seeds
Abstract
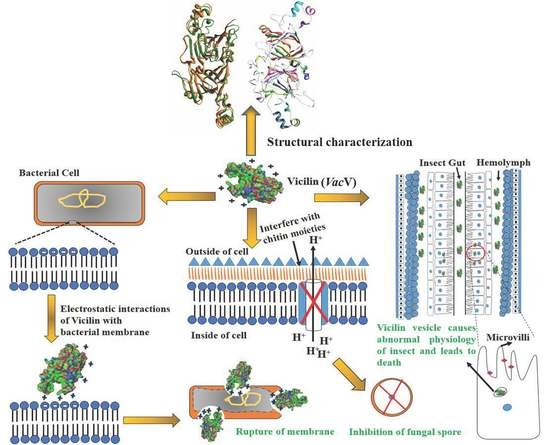
:1. Introduction
2. Results
2.1. Purification of VacV
2.2. Protein Identification by LC-MS/MS Spectrometry
2.3. VacV Structure Prediction and Phylogenetic Analysis
2.4. Antibacterial Activity Assay
2.5. Antifungal Assessment Activity
2.6. Determination of Insecticidal Activity
2.7. Effect of VacV on Life Stage of S. oryzae
2.8. Evaluation of Cell Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Biological Materials and Growth Conditions
4.2. Isolation and Purification of VacV
4.3. Characterization of the Purified Protein
LC-MS/MS Mass Spectrometry Analysis
4.4. Bioinformatics Analysis
4.4.1. Multiple Sequence Alignment and Evolutionary Assessment
4.4.2. Molecular Modelling and Docking
4.5. Biological Assays
4.5.1. Antibacterial Activity
4.5.2. Antifungal Assessment
4.5.3. Determination of Insecticidal Activity
4.5.4. Assessment of Anticancer Activity toward HepG-2 Cancerous Cell Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Food and Agricultural Organization of the United Nations FAOSTAT Crops. Available online: http://www.fao.org/ (accessed on 27 February 2022).
- Zaheer, M.; Ahmed, S.; Hassan, M.M. Vigna unguiculata (L.) Walp.(Papilionaceae): A review of medicinal uses, Phytochemistry and pharmacology. J. Pharmacogn. Phytochem. 2020, 9, 1349–1352. [Google Scholar]
- Effa, E.A. Evaluating the Effect of Organic Manure and Agricultural Lime on Chlorophyll and Oxidative Stress Enzymes of Dry Bean (Phaseolus vulgaris) and Moth Bean (Vigna aconitifolia). Asian J. Res. Bot. 2020, 3, 18–26. [Google Scholar]
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shivhare, U.S.; Vishwakarma, R.K. Antinutritional compounds in pulses: Implications and alleviation methods. Legum. Sci. 2021, e111. [Google Scholar] [CrossRef]
- Opara, C.; Egbuonu, A.; Obike, C. Assessment of Proximate, Vitamins, Minerals and Antinutrients Compositions of Unprocessed Vigna aconitifolia (Moth Bean) Seeds. Arch. Curr. Res. Int. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Kamani, M.H.; Martin, A.; Meera, M.S. Valorization of By-products Derived from Milled Moth Bean: Evaluation of Chemical Composition, Nutritional Profile and Functional Characteristics. Waste Biomass Valorization 2020, 11, 4895–4906. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Monteiro, S.V.; Carrapiço, B.M.; Ferreira, R.B. Are vicilins another major class of legume lectins? Molecules 2014, 19, 20350–20373. [Google Scholar] [CrossRef] [Green Version]
- Bennetau-Pelissero, C. Plant Proteins from Legumes; Springer: Cham, Switzerland, 2019; ISBN 9783319545288. [Google Scholar]
- Kesari, P.; Neetu, B.S.P.; Sharma, A.; Katiki, M.; Kumar, P.; Gurjar, B.; Tomar, S.; Sharma, A.; Kumar, P. Structural, Functional and Evolutionary Aspects of Seed Globulins. Protein Pept. Lett. 2017, 24, 267–277. [Google Scholar] [CrossRef]
- Kumar, P.; Tomar, S.; Sharma, A.; Gurjar, B.; Singh, P.; Mishra, M.; Katiki, M.; Neetu, B.S.P.; Kesari, P.; Kumar, P.; et al. Purification and Characterization of 2S Albumin from Seeds of Wrightia tinctoria Exhibiting Antibacterial and DNase Activity. Protein Pept. Lett. 2017, 24, 368–378. [Google Scholar] [CrossRef]
- Sathe, S.K.; Venkatachalam, M. Fractionation and biochemical characterization of moth bean (Vigna aconitifolia L.) proteins. LWT Food Sci. Technol. 2007, 40, 600–610. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C. Narrow-leafed lupin (Lupinus angustifolius L.) β-conglutin: A multifunctional family of proteins with roles in plant defence, human health benefits, and potential uses as functional food. Legum. Sci. 2020, 2, e33. [Google Scholar] [CrossRef] [Green Version]
- Adsule, R.N. Moth bean (Vigna aconitifolia (Jacq.) Maréchal). Food Feed from Legum. Oilseeds 1996, 203–205. [Google Scholar] [CrossRef]
- Warsame, A.O.; O’Sullivan, D.M.; Tosi, P. Seed Storage Proteins of Faba Bean (Vicia faba L): Current Status and Prospects for Genetic Improvement. J. Agric. Food Chem. 2018, 66, 12617–12626. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.J.; Costa, J.H.; Paiva, A.L.S.; de Oliveira Barsottini, M.R. Genome-wide analysis of the cupin superfamily in cowpea (Vigna unguiculata). Plant Gene 2021, 25, 100265. [Google Scholar] [CrossRef]
- McPherson, A. Binding of benzoic acid and anions within the cupin domains of the vicilin protein canavalin from jack bean (Canavalia ensiformis): Crystal structures. Biochem. Biophys. Res. Commun. 2020, 524, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef]
- Xie, Z.; Saha, N.; Chlan, C. Antimicrobial Activity of a Cys-Rich Peptide Derived from a Centrosema virginianum Vicilin. Am. J. Plant Sci. 2016, 07, 92–107. [Google Scholar] [CrossRef]
- Saad, A.M.; Elmassry, R.A.; Wahdan, K.M.; Osman, A.O. Chemical characterization, antibacterial and antioxidant activity of 7s and 11s globulins isolated from pea (Pisum sativum). Zagazig J. Agric. Res. 2017, 44, 2221–2230. [Google Scholar] [CrossRef]
- Rocha, A.J.; Sousa, B.L.; Girão, M.S.; Barroso-Neto, I.L.; Monteiro-Júnior, J.E.; Oliveira, J.T.A.; Nagano, C.S.; Carneiro, R.F.; Monteiro-Moreira, A.C.O.; Rocha, B.A.M.; et al. Cloning of cDNA sequences encoding cowpea (Vigna unguiculata) vicilins: Computational simulations suggest a binding mode of cowpea vicilins to chitin oligomers. Int. J. Biol. Macromol. 2018, 117, 565–573. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Enan, G. Characterization and Antibacterial Activity of 7S and 11S Globulins Isolated from Cowpea Seed Protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef] [Green Version]
- Yunes, A.N.A.; de Andrade, M.T.; Sales, M.P.; Morais, R.A.; Fernandes, K.V.S.; Gomes, V.M.; Xavier-Filho, J. Legume seed vicilins (7S storage proteins) interfere with the development of the cowpea weevil (Callosobruchus maculatus (F)). J. Sci. Food Agric. 1998, 76, 111–116. [Google Scholar] [CrossRef]
- Osman, A.; Abbas, E.; Mahgoub, S.; Sitohy, M. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J. Gen. Plant Pathol. 2016, 82, 293–301. [Google Scholar] [CrossRef]
- Bernardo, A.E.N.; Garcia, R.N.; Adachi, M.; Angeles, J.G.C.; Kaga, A.; Ishimoto, M.; Utsumi, S.; Tecson-Mendoza, E.M. 8S globulin of mungbean [Vigna radiata (L.) Wilczek]: Cloning and characterization of its cDNA isoforms, expression in Escherichia coli, purification, and crystallization of the major recombinant 8S isoform. J. Agric. Food Chem. 2004, 52, 2552–2560. [Google Scholar] [CrossRef]
- Freitas, R.L.; Teixeira, A.R.; Ferreira, R.B. Characterization of the Proteins from Vigna unguiculata Seeds. J. Agric. Food Chem. 2004, 52, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Imran, M.; Ali, A.; Munawar, A.; Khaliq, B.; Anwar, F.; Saeed, Q.; Buck, F.; Hussain, S.; Saeed, A.; et al. Model prediction of a kunitz-type trypsin inhibitor protein from seeds of acacia nilotica l. With strong antimicrobial and insecticidal activity. Turkish J. Biol. 2020, 44, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Jain, E.; Bairoch, A.; Duvaud, S.; Phan, I.; Redaschi, N.; Suzek, B.E.; Martin, M.J.; McGarvey, P.; Gasteiger, E. Infrastructure for the life sciences: Design and implementation of the UniProt website. BMC Bioinformatics 2009, 10, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Y.S.; Xia, L.; Ng, T.B. White kidney bean lectin exerts anti-proliferative and apoptotic effects on cancer cells. Int. J. Biol. Macromol. 2016, 85, 335–345. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, Z.; Jiang, P.; Zhang, R.; Ni, D.; Yuan, Y.; Zhang, S. Characterization of chikusetsusaponin IV and V induced apoptosis in HepG2 cancer cells. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef]
- Garino, C.; De Paolis, A.; Coïsson, J.D.; Arlorio, M. Pru du 2S albumin or Pru du vicilin? Comput. Biol. Chem. 2015, 56, 30–32. [Google Scholar] [CrossRef]
- Rangel, A.; Domont, G.B.; Pedrosa, C.; Ferreira, S.T. Functional properties of purified vicilins from cowpea (Vigna unguiculata) and pea (Pisum sativum) and cowpea protein isolate. J. Agric. Food Chem. 2003, 51, 5792–5797. [Google Scholar] [CrossRef]
- Croy, R.R.; Gatehouse, J.A.; Tyler, M.; Boulter, D. The purification and characterization of a third storage protein (convicilin) from the seeds of pea (Pisum sativum L.). Biochem. J. 1980, 191, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Albillos, S.M.; Chen, Y.-W.; Kothary, M.H.; Fu, T.-J.; Zhang, Y.-Z. Purification and characterization of the 7S vicilin from Korean pine (Pinus koraiensis). J. Agric. Food Chem. 2008, 56, 8159–8165. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.M.T.; Adachi, M.; Bernardo, A.E.N.; Utsumi, S. Mungbean [Vigna radiata (L.) Wilczek] globulins: Purification and characterization. J. Agric. Food Chem. 2001, 49, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Prak, K.; Fujioka, M.; Maruyama, N.; Utsumi, S. Physicochemical properties of native adzuki bean (Vigna angularis) 7S globulin and the molecular cloning of its cDNA isoforms. J. Agric. Food Chem. 2007, 55, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.; Oliveira, G.B.; Brascher, T.C.; Samuels, R.I.; Macedo, M.L.R.; de Souza, L.F.; Dafré, A.L.; Silva, C.P. Phaseolin ingestion affects vesicular traffic causing oxidative stress in the midgut of Callosobruchus maculatus larvae. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 228, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Y.; Shi, R.; Chen, Z.; Li, Z.; Wei, Y.; Zhou, X. Structural and antioxidant analysis of Tartary buckwheat (Fagopyrum tartaricum Gaertn.) 13S globulin. J. Sci. Food Agric. 2020, 100, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sathe, S.K.; Su, M.; Liu, C. Germination reduces black gram (Vigna mungo) and mung bean (Vigna radiata) vicilin immunoreactivity. Lwt 2021, 135, 110217. [Google Scholar] [CrossRef]
- Kimura, A.; Tandang-Silvas, M.R.G.; Fukuda, T.; Cabanos, C.; Takegawa, Y.; Amano, M.; Nishimura, S.-I.; Matsumura, Y.; Utsumi, S.; Maruyama, N. Carbohydrate moieties contribute significantly to the physicochemical properties of French bean 7S globulin phaseolin. J. Agric. Food Chem. 2010, 58, 2923–2930. [Google Scholar] [CrossRef]
- Kesari, P.; Pratap, S.; Dhankhar, P.; Dalal, V.; Mishra, M.; Singh, P.K.; Chauhan, H.; Kumar, P. Structural characterization and in-silico analysis of Momordica charantia 7S globulin for stability and ACE inhibition. Sci. Rep. 2020, 10, 1160. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Mattison, C.P.; Zhang, D.; Grimm, C.C. Vicilin and legumin storage proteins are abundant in water and alkali soluble protein fractions of glandless cottonseed. Sci. Rep. 2021, 11, 9209. [Google Scholar] [CrossRef]
- Sousa Silva, C.R.; Figueira, A. Phylogenetic analysis of Theobroma (Sterculiaceae) based on Kunitz-like trypsin inhibitor sequences. Plant Syst. Evol. 2005, 250, 93–104. [Google Scholar] [CrossRef]
- Kratzer, U.; Frank, R.; Kalbacher, H.; Biehl, B.; Wöstemeyer, J.; Voigt, J. Subunit structure of the vicilin-like globular storage protein of cocoa seeds and the origin of cocoa- and chocolate-specific aroma precursors. Food Chem. 2009, 113, 903–913. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant pep tides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Szerszunowicz, I.; Kłobukowski, J. Characteristics of potential protein nutraceuticals of plant origin with antioxidant activity. Molecules 2020, 25, 1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartal, C.; Kaplan Türköz, B.; Otles, S. Prediction, identification and evaluation of bioactive peptides from tomato seed proteins using in silico approach. J. Food Meas. Charact. 2020, 14, 1865–1883. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Frias, J.; Peñas, E.; Zieliński, H.; Giménez-Bastida, J.A.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. J. Funct. Foods 2015, 18, 319–332. [Google Scholar] [CrossRef] [Green Version]
- de Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejía, E.G. Impact of germination and enzymatic hydrolysis of cowpea bean (Vigna unguiculata) on the generation of peptides capable of inhibiting dipeptidyl peptidase IV. Food Res. Int. 2014, 64, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Savinase, the most suitable enzyme for releasing peptides from lentil (Lens culinaris var. Castellana) protein concentrates with multifunctional properties. J. Agric. Food Chem. 2014, 62, 4166–4174. [Google Scholar] [CrossRef] [Green Version]
- Al Saiqali, M.; Tangutur, A.D.; Banoth, C.; Bhukya, B. Antimicrobial and anticancer potential of low molecular weight polypeptides extracted and characterized from leaves of Azadirachta indica. Int. J. Biol. Macromol. 2018, 114, 906–921. [Google Scholar] [CrossRef]
- Jain, A.; Kumar, A.; Salunke, D.M. Crystal structure of the vicilin from Solanum melongena reveals existence of different anionic ligands in structurally similar pockets. Sci. Rep. 2016, 6, 23600. [Google Scholar] [CrossRef] [Green Version]
- Viega de Andrade, E.K.; Rodrigues, R.; da Costa Vieira Bard, G.; da Silva Pereira, L.; Ventury Baptista, K.E.; Menezes Cavalcanti, T.F.; Amâncio Oliveira, A.E.; Melo Souza, T.A.; Gomes, V.M. Identification, biochemical characterization and biological role of defense proteins from common bean genotypes seeds in response to Callosobruchus maculatus infestation. J. Stored Prod. Res. 2020, 87, 101580. [Google Scholar] [CrossRef]
- Moura, F.T.; Oliveira, A.S.; Macedo, L.L.P.; Vianna, A.L.B.R.; Andrade, L.B.S.; Martins-Miranda, A.S.; Oliveira, J.T.A.; Santos, E.A.; De Sales, M.P. Effects of a chitin-binding vicilin from Enterolobium contortisiliquum seeds on bean bruchid pests (Callosobruchus maculatus and Zabrotes subfasciatus) and phytopathogenic fungi (Fusarium solani and Colletrichum lindemuntianum). J. Agric. Food Chem. 2007, 55, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Bard, G.C.V.; Nascimento, V.V.; Oliveira, A.E.A.; Rodrigues, R.; Da Cunha, M.; Dias, G.B.; Vasconcelos, I.M.; Carvalho, A.O.; Gomes, V.M. Vicilin-like peptides from Capsicum baccatum L. seeds are α-amylase inhibitors and exhibit antifungal activity against important yeasts in medical mycology. Biopolym. Pept. Sci. Sect. 2014, 102, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.M.; Mosqueda, M.I.; Blanco-Labra, A.; Sales, M.P.; Fernandes, K.V.S.; Cordeiro, R.A.; Xavier-Filho, J. Vicilin Storage Proteins from Vigna unguiculata (Legume) Seeds Inhibit Fungal Growth. J. Agric. Food Chem. 1997, 45, 4110–4115. [Google Scholar] [CrossRef]
- Shikhi, M.; Nair, D.T.; Salunke, D.M. Structure-guided identification of function: Role of Capsicum annuum vicilin during oxidative stress. Biochem. J. 2018, 475, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Browne, J.J.; Van Crugten, J.; Hasan, M.F.; Liu, L.; Barkla, B.J. In Silico, Molecular Docking and In Vitro Antimicrobial Activity of the Major Rapeseed Seed Storage Proteins. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Mahgoub, S.A.; Osman, A.O. In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int. J. Food Microbiol. 2012, 154, 19–29. [Google Scholar] [CrossRef]
- Afroz, M.; Akter, S.; Ahmed, A.; Rouf, R.; Shilpi, J.A.; Tiralongo, E.; Sarker, S.D.; Göransson, U.; Uddin, S.J. Ethnobotany and Antimicrobial Peptides From Plants of the Solanaceae Family: An Update and Future Prospects. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim. Biophys. Acta Biomembr. 2015, 1848, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.F.F.; Agizzio, A.P.; Machado, O.L.T.; Neves-Ferreira, A.G.C.; Oliveira, M.A.; Fernandes, K.V.S.; Carvalho, A.O.; Perales, J.; Gomes, V.M. A new peptide of melon seeds which shows sequence homology with vicilin: Partial characterization and antifungal activity. Sci. Hortic. 2007, 111, 399–405. [Google Scholar] [CrossRef]
- Miranda, M.R.A.; Uchôa, A.F.; Ferreira, S.R.; Ventury, K.E.; Costa, E.P.; Carmo, P.R.L.; Machado, O.L.T.; Fernandes, K.V.S.; Amancio Oliveira, A.E. Chemical Modifications of Vicilins Interfere with Chitin-Binding Affinity and Toxicity to Callosobruchus maculatus (Coleoptera: Chrysomelidae) Insect: A Combined In Vitro and In Silico Analysis. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Souza, A.J.; Ferreira, A.T.S.; Perales, J.; Beghini, D.G.; Fernandes, K.V.S.; Xavier-Filho, J.; Venancio, T.M.; Oliveira, A.E.A. Identification of Albizia lebbeck seed coat chitin-binding vicilins (7S globulins) with high toxicity to the larvae of the bruchid Callosobruchus maculatus. Braz. J. Med. Biol. Res. 2012, 45, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- França, A.F.J.; Araújo, J.N.; Santos, Y.Q.; Carelli, G.S.C.; Silva, D.A.; Amorim, T.M.L.; Migliolo, L.; Santos, E.A.; Oliveira, A.S.; Uchôa, A.F. Vicilin from Anadenanthera colubrina Seeds: An alternative tool to combat Callosobruchus maculatus. Saudi J. Biol. Sci. 2021, 28, 5229–5237. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Rafiq, Z.; Saeed, Q.; Khaliq, B.; Ullah, A.; Mehmood, S.; Ali, Z.; Ashraf, M.Y.; Akrem, A. Functional characterization of a potent antimicrobial and insecticidal chitin binding protein from seeds of Iberis umbellata L. Pak. J. Bot. 2021, 53, 1515–1523. [Google Scholar] [CrossRef]
- Atallah, O.O.; Osman, A.; Ali, M.A.S.; Sitohy, M. Soybean β-conglycinin and catfish cutaneous mucous p22 glycoproteins deteriorate sporangial cell walls of Pseudoperonospora cubensis and suppress cucumber downy mildew. Pest Manag. Sci. 2021, 77, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sabour, A.G.; Obiadalla-Ali, H.A.; Abdelrehim, K.A. Genetic and chemical analyses of six cowpea and two Phaseolus bean species differing in resistance to weevil pest. J. Crop Sci. Biotechnol. 2010, 13, 53–60. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Yan, L.; Cheng, X.; Kang, Y. Functional properties of 8S globulin fractions from 15 mung bean (Vigna radiata (L.) Wilczek) cultivars. Int. J. Food Sci. Technol. 2015, 50, 1206–1214. [Google Scholar] [CrossRef]
- Mota, A.C.; DaMatta, R.A.; Filho, M.L.; Silva, C.P.; Xavier-Filho, J. Cowpea (Vigna unguiculata) vicilins bind to the peritrophic membrane of larval sugarcane stalk borer (Diatraea saccharalis). J. Insect Physiol. 2003, 49, 873–880. [Google Scholar] [CrossRef]
- Alexandre, D.; Linhares, R.T.; Queiroz, B.; Fontoura, L.; Uchôa, A.F.; Samuels, R.I.; Macedo, M.L.R.; Bezerra, C.S.; Oliveira, E.M.; Demartini, D.R.; et al. Vicilin-derived peptides are transferred from males to females as seminal nuptial gift in the seed-feeding beetle Callosobruchus maculatus. J. Insect Physiol. 2011, 57, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Paes, E.V.; Uchôa, A.F.; Pinto, M.S.T.; Silva, C.P.; Fernandes, K.V.S.; Oliveira, A.E.A.; Xavier-Filho, J. Binding of Vigna unguiculata vicilins to the peritrophic membrane of Tenebrio molitor affects larval development. Entomol. Exp. Appl. 2008, 129, 11–17. [Google Scholar] [CrossRef]
- de Sales, M.P.; Maria, M.L.; Xavier-Filho, J. Digestibility of cowpea (Vigna unguiculata) vicilins by pepsin, papain and bruchid (insect) midgut proteinases. Comp. Biochem. Physiol. -Part B Biochem. 1992, 103, 945–950. [Google Scholar] [CrossRef]
- Gupta, N.; Srivastava, N.; Bhagyawant, S.S. Vicilin—A major storage protein of mungbean exhibits antioxidative potential, antiproliferative effects and ACE inhibitory activity. PLoS ONE 2018, 13, e0191265. [Google Scholar] [CrossRef] [PubMed]
- LAEMMLI, U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nat. Publ. Gr. 1970, 228, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Carlsson, N.; Borde, A.; Wölfel, S.; Kerman, B.; Larsson, A. Quantification of protein concentration by the Bradford method in the presence of pharmaceutical polymers. Anal. Biochem. 2011, 411, 116–121. [Google Scholar] [CrossRef]
- Ponzoni, E.; Brambilla, I.M.; Galasso, I. Genome-wide identification and organization of seed storage protein genes of Cannabis sativa. Biol. Plant. 2018, 62, 693–702. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C.; Zafra, A.; Palanco, L.; Florido, J.F.; Alché, J.D.D. Identification and assessment of the potential allergenicity of 7S vicilins in olive (Olea europaea L.) seeds. Biomed Res. Int. 2016, 2016, 4946872. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinform. 2007, 50. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.W.; Leaver-Fay, A.; Chen, V.B.; Block, J.N.; Kapral, G.J.; Wang, X.; Murray, L.W.; Arendall, W.B.; Snoeyink, J.; Richardson, J.S.; et al. MolProbity: All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007, 35, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.Y. HDOCK: A web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.A.; Akter, Z.; Uddin, M.J.; Ferdaus, K.M.K.B.; Hoque, K.M.F.; Ferdousi, Z.; Reza, M.A. Characterization and evaluation of antibacterial and antiproliferative activities of crude protein extracts isolated from the seed of Ricinus communis in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 211. [Google Scholar] [CrossRef] [Green Version]
- Boyle, V.J.; Fancher, M.E.; Ross, R.W. Rapid, modified Kirby-Bauer susceptibility test with single, high-concentration antimicrobial disks. Antimicrob. Agents Chemother. 1973, 3, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Teodorowicz, M.; Fiedorowicz, E.; Kostyra, H.; Wichers, H.; Kostyra, E. Effect of Maillard reaction on biochemical properties of peanut 7S globulin (Ara h 1) and its interaction with a human colon cancer cell line (Caco-2). Eur. J. Nutr. 2013, 52, 1927–1938. [Google Scholar] [CrossRef] [Green Version]









| Purification Steps | Total Protein (mg) | Purification (times) | Recovery Yield (%) |
|---|---|---|---|
| Crude | 350 | 1 | 100 |
| Ammonium sulfate fractionation (60%) | 245 | 1.42 | 70 |
| Hi-Load 16/60 Superdex | 122.5 | 2.85 | 35 |
| Hi trap Q FF C | 12.2 | 28.6 | 3.48 |
| Sr. No. | Peptide Sequences (25 kDa) | |
|---|---|---|
| 1 | GKNNPFYFNSDR (12) | |
| 2 | QMQNLENYR (09) | |
| 3 | AVLTLVNPDGGR (12) | |
| 4 | IPAGTIFFLVNPDDNENLRIIKLAVPVNNPHRFQDFFLSSTEAQQSYLQGFSKNILEASFDSDIKEINRVLFGEEGQQQQQGQESQQEGVIVELKR (96) | |
| 5 | SLSSEDQPFN (10) | |
| 6 | DLDVFISSVDMKEGSLLLPHYNSKAIVILVINEGEANIELVGL (43) | |
| 7 | NFLAGEKDNVISEIPTEVLDLAFPAPGEKVEK (32) | |
| Sr. No. | Peptide Sequences (30 kDa) | Peptide Sequences (40 kDa) |
| 1 | LSYFVDAQPQQK (12) | TVSSEDEPFNLR (12) |
| 2 | VLEVAFPGSVSK (12) | NPAGTLFFLVNPDDNENLR (19) |
| 3 | TVSSQNEPFNLR (12) | FQDFFLSSTEAQQSYLQGFSK (21) |
| Sr. No. | Peptide Sequences (50 kDa) | |
| 1 | GKNNPFYFNSDR (12) | |
| 2 | SKQMQNLENYR (11) | |
| 3 | AVLTLVNPDGGR (12) | |
| 4 | AGTIFFLVNPDDNENLRIIKLAVPVNNPHRFQDFFLSSTEAQQSYLQGFSKNILEASFDASDIKEINRVLFGEEGQQQQQGQESQQEGVIVELKR (95) | |
| 5 | SSSKKSLSSEDQPFNLR (17) | |
| 6 | NPQLKDLDVFISSVDMKEGSLLLPHYNSKAIVILVINEGEANIELVGLR (49) | |
| 7 | FGINAENNQRNFLAGEKDNVISEIPTEVLDLAFPAPGEKVEK (42) | |
| Plants | Sequence | Protein Name | Homology (%) | Accession No. |
|---|---|---|---|---|
| V. aconitifolia | GKNNPFYFNSDR | VacV | 100 | This Study |
| V. radiata | GKNNPFYFNSDR | 8Sα | 100 | A0A3P9QP39 |
| V. angularis | GKNNPFYFNSDR | VanV | 100 | A0A0L9U0Y5 |
| V. unguiculata | GQNNPFYFDSDR | Vicilin | 83.3 | A8YQH5 |
| Protein | Bacteria | Inhibition Zone Diameter (mm) | ||
|---|---|---|---|---|
| VacV 15 μg/disc | VacV 30 μg/disc | Antibiotic 5 μg/disc | ||
| VacV | Bacillus subtilis | 15 ± 2 | 18 ± 2 | 28 ± 1 |
| VacV | Escherichia coli | 13 ± 2 | 17 ± 2 | 27 ± 2 |
| VacV | Pseudomonas aeruginosa | 16 ± 2 | 20 ± 3 | 30 ± 1 |
| VacV | Staphylococcus aureus | 11 ± 2 | 16 ± 2 | 22 ± 2 |
| VacV | Xanthomonas oryzae | 18 ± 2 | 21 ± 1 | 31 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateeq, M.; Adeel, M.M.; Kanwal, A.; Tahir ul Qamar, M.; Saeed, A.; Khaliq, B.; Saeed, Q.; Atiq, M.N.; Bilal, M.; Alharbi, M.; et al. In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna aconitifolia (Jacq.) Marechal) Seeds. Molecules 2022, 27, 3251. https://doi.org/10.3390/molecules27103251
Ateeq M, Adeel MM, Kanwal A, Tahir ul Qamar M, Saeed A, Khaliq B, Saeed Q, Atiq MN, Bilal M, Alharbi M, et al. In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna aconitifolia (Jacq.) Marechal) Seeds. Molecules. 2022; 27(10):3251. https://doi.org/10.3390/molecules27103251
Chicago/Turabian StyleAteeq, Muhammad, Muhammad Muzammal Adeel, Ayesha Kanwal, Muhammad Tahir ul Qamar, Ahsan Saeed, Binish Khaliq, Qamar Saeed, Muhammad Nauman Atiq, Muhammad Bilal, Metab Alharbi, and et al. 2022. "In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna aconitifolia (Jacq.) Marechal) Seeds" Molecules 27, no. 10: 3251. https://doi.org/10.3390/molecules27103251