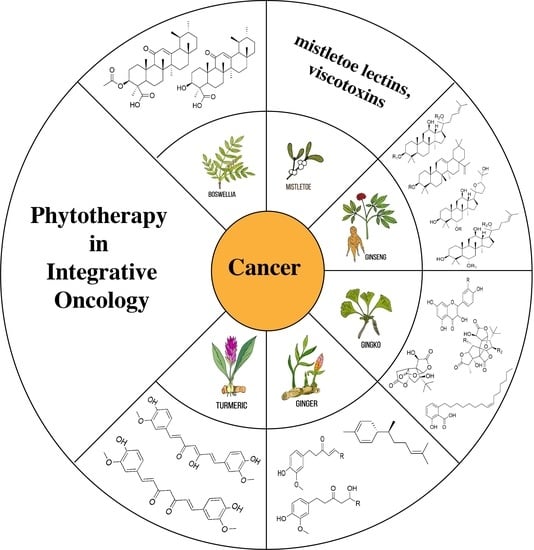
Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options
Abstract
:1. Introduction
2. Search Strategy
3. Plants and Their Ingredients in Oncology
4. Boswellia/Frankincense
5. Mistletoe (Viscum album L.)
6. Gingko
7. Ginseng
8. Ginger
9. Curcumin
10. Risks, Side Effects and Interactions
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMG | Medicinal Products Act |
| CNS | Central Nervous System |
| EMA | European Medicines Agency |
| ESCOP | European Scientific Cooperative on Phytotherapy |
| HMP | Herbal Medicinal Products |
| HMPC | Committee on Herbal Medicinal Products |
| mRNA | Messenger Ribonucleic Acid |
| TCM | Traditional Chinese Medicine |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Loftfield, E. Cancer Epidemiology: A Survey of Modifiable Risk Factors for Prevention and Survivorship. Am. J. Lifestyle Med. 2018, 12, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical complications in colorectal cancer patients. Ann. Med. Surg. 2020, 55, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kroschinsky, F.; Stölzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, R.; Brown, J. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb Perspect. Med. 2018, 8, a033415. [Google Scholar] [CrossRef]
- Sarmento-Ribeiro, A.B.; Scorilas, A.; Gonçalves, A.C.; Efferth, T.; Trougakos, I.P. The emergence of drug resistance to targeted cancer therapies: Clinical evidence. Drug Resist. Update 2019, 47, 100646. [Google Scholar] [CrossRef]
- Lemonnier, N.; Zhou, G.-B.; Prasher, B.; Mukerji, M.; Chen, Z.; Brahmachari, S.K.; Noble, D.; Auffray, C.; Sagner, M. Traditional Knowledge-based Medicine: A Review of History, Principles, and Relevance in the Present Context of P4 Systems Medicine. Prog. Prev. Med. 2017, 2, e0011. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Appendino, G.; Fontana, G.; Pollastro, F. Natural Products Drug Discovery. Compr. Nat. Prod. II Chem. Biol. 2010, 3, 205–236. [Google Scholar]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Bäumler, S. Heilpflanzenpraxis. Heute: Porträts-Rezepturen-Anwendung; Urban & Fischer bei Elsevier: München, Germany, 2006. [Google Scholar]
- Kingston, D.G.I. Modern Natural Products Drug Discovery and Its Relevance to Biodiversity Conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Gokduman, K. Strategies Targeting DNA Topoisomerase I in Cancer Chemotherapy: Camptothecins, Nanocarriers for Camptothecins, Organic Non-Camptothecin Compounds and Metal Complexes. Curr. Drug Targets 2016, 17, 1928–1939. [Google Scholar] [CrossRef]
- Asiimwe, J.B.; Nagendrappa, P.B.; Atukunda, E.C.; Kamatenesi, M.M.; Nambozi, G.; Tolo, C.U.; Ogwang, P.E.; Sarki, A.M. Prevalence of the Use of Herbal Medicines among Patients with Cancer: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2021, 2021, 9963038. [Google Scholar] [CrossRef]
- Damery, S.; Gratus, C.; Grieve, R.; Warmington, S.; Jones, J.; Routledge, P.; Greenfield, S.; Dowswell, G.; Sherriff, J.; Wilson, S. The use of herbal medicines by people with cancer: A cross-sectional survey. Br. J. Cancer 2011, 104, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Ocran Mattila, P.; Ahmad, R.; Hasan, S.S.; Babar, Z.-U.-D. Availability, Affordability, Access, and Pricing of Anti-cancer Medicines in Low- and Middle-Income Countries: A Systematic Review of Literature. Front. Public Health 2021, 9, 462. [Google Scholar] [CrossRef]
- Katzke, V.A.; Kaaks, R.; Kühn, T. Lifestyle and cancer risk. Cancer J. 2015, 21, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Giovannucci, E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors among White Adults in the United States. JAMA Oncol. 2016, 2, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Vijayvergia, N.; Denlinger, C.S. Lifestyle Factors in Cancer Survivorship: Where We Are and Where We Are Headed. J. Pers. Med. 2015, 5, 243–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenigmann, M. Phytotherapie: Arzneidrogen Phytopharmka Anwendung; Elsevier Health Sciences/Urban & Fischer, Elsevier: Munich, Germany, 2017. [Google Scholar]
- Schilcher, H.; Kammerer, S.; Wegener, T.; Volkmann, D. (Eds.) Kapitel 1—Grundlegendes zur rationalen Phytotherapie. In Leitfaden Phytotherapie (Dritte Ausgabe); Urban & Fischer: Munich, Germany, 2007; pp. 1–30. [Google Scholar]
- European Scientific Cooperative on Phytotherapy. Available online: https://escop.com/about-escop/ (accessed on 1 April 2022).
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Ernst, E. Harmless herbs? A review of the recent literature. Am. J. Med. 1998, 104, 170–178. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, B.; Tulsyan, S.; Kumar, S.; Mittal, R.D.; Agarwal, G. Cytochrome P450 in Cancer Susceptibility and Treatment. Adv. Clin. Chem. 2015, 71, 77–139. [Google Scholar] [PubMed]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction with Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özenver, N.; Efferth, T. Integration of Phytochemicals and Phytotherapy into Cancer Precision Medicine. In Approaching Complex Diseases: Network-Based Pharmacology and Systems Approach in Bio-Medicine; Bizzarri, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 355–392. [Google Scholar]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency European Union Monographs and List Entries. Available online: https://www.ema.europa.eu/en/human-regulatory/herbal-products/european-union-monographs-list-entries (accessed on 1 April 2022).
- European Scientific Cooperative on Phytotherapy about the Scientific Committee. Available online: https://escop.com/about-escop/scientific-committee/ (accessed on 1 April 2022).
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- WHO. Consultation on Selected Medicinal Plants, WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- McCune, J.S.; Hatfield, A.J.; Blackburn, A.A.; Leith, P.O.; Livingston, R.B.; Ellis, G.K. Potential of chemotherapy-herb interactions in adult cancer patients. Support Care Cancer 2004, 12, 454–462. [Google Scholar] [CrossRef]
- Ashraf, M.A. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty. BioMed Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Shah, N.R.; Patel, B.M. Secoisolariciresinol diglucoside rich extract of L. usitatissimum prevents diabetic colon cancer through inhibition of CDK4. Biomed. Pharm. 2016, 83, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Iqbal, R.; Safdar, M.; Murtaza, S.; Mustafa, G.; Sajjad, M.; Bukhari, S.A.; Huma, T. Antioxidant and antibacterial activities of Artemisia absinthium and Citrus paradisi extracts repress viability of aggressive liver cancer cell line. Mol. Biol. Rep. 2021, 48, 7703–7710. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.A.; Richmond, R.C.; Santos Ferreira, D.L.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Würtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The ProDiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine 2015, 94, e1260. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Beretta, G.L. Resveratrol and Prostate Cancer: The Power of Phytochemicals. Curr. Med. Chem. 2021, 28, 4845–4862. [Google Scholar] [CrossRef]
- Heggie, S.; Bryant, G.P.; Tripcony, L.; Keller, J.; Rose, P.; Glendenning, M.; Heath, J. A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002, 25, 442–451. [Google Scholar] [CrossRef]
- Hoopfer, D.; Holloway, C.; Gabos, Z.; Alidrisi, M.; Chafe, S.; Krause, B.; Lees, A.; Mehta, N.; Tankel, K.; Strickland, F.; et al. Three-Arm Randomized Phase III Trial: Quality Aloe and Placebo Cream Versus Powder as Skin Treatment During Breast Cancer Radiation Therapy. Clin. Breast Cancer 2015, 15, 181–190.e4. [Google Scholar] [CrossRef]
- Olsen, D.L.; Raub, W., Jr.; Bradley, C.; Johnson, M.; Macias, J.L.; Love, V.; Markoe, A. The effect of aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol. Nurs. Forum. 2001, 28, 543–547. [Google Scholar]
- Williams, M.S.; Burk, M.; Loprinzi, C.L.; Hill, M.; Schomberg, P.J.; Nearhood, K.; O’Fallon, J.R.; Laurie, J.A.; Shanahan, T.G.; Moore, R.L.; et al. Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 345–349. [Google Scholar] [CrossRef]
- Mansouri, P.; Haghighi, M.; Beheshtipour, N.; Ramzi, M. The Effect of Aloe Vera Solution on Chemotherapy-Induced Stomatitis in Clients with Lymphoma and Leukemia: A Randomized Controlled Clinical Trial. Int. J. Community Based Nurs. Midwifery 2016, 4, 119–126. [Google Scholar] [PubMed]
- Saberi-Karimian, M.; Katsiki, N.; Caraglia, M.; Boccellino, M.; Majeed, M.; Sahebkar, A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 299–312. [Google Scholar] [CrossRef]
- da Costa Miranda, V.; Trufelli, D.C.; Santos, J.; Campos, M.P.; Nobuo, M.; da Costa Miranda, M.; Schlinder, F.; Riechelmann, R.; del Giglio, A. Effectiveness of guaraná (Paullinia cupana) for postradiation fatigue and depression: Results of a pilot double-blind randomized study. J. Altern. Complement. Med. 2009, 15, 431–433. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Campos, M.P.; Riechelmann, R.; Martins, L.C.; Hassan, B.J.; Casa, F.B.; Del Giglio, A. Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J. Altern. Complement. Med. 2011, 17, 505–512. [Google Scholar] [CrossRef]
- del Giglio, A.B.; Cubero Dde, I.; Lerner, T.G.; Guariento, R.T.; de Azevedo, R.G.; Paiva, H.; Goldman, C.; Carelli, B.; Cruz, F.M.; Schindler, F.; et al. Purified dry extract of Paullinia cupana (guaraná) (PC-18) for chemotherapy-related fatigue in patients with solid tumors: An early discontinuation study. J. Diet Suppl. 2013, 10, 325–334. [Google Scholar] [CrossRef]
- Martins, S.; Ferreira, C.L.; Del Giglio, A. Placebo-Controlled, Double-Blind, Randomized Study of a Dry Guarana Extract in Patients with Head and Neck Tumors Undergoing Chemoradiotherapy: Effects on Fatigue and Quality of Life. J. Diet Suppl. 2017, 14, 32–41. [Google Scholar] [CrossRef]
- Barton, D.L.; Atherton, P.J.; Bauer, B.A.; Moore, D.F., Jr.; Mattar, B.I.; Lavasseur, B.I.; Rowland, K.M., Jr.; Zon, R.T.; Lelindqwister, N.A.; Nagargoje, G.G.; et al. The use of Valeriana officinalis (Valerian) in improving sleep in patients who are undergoing treatment for cancer: A phase III randomized, placebo-controlled, double-blind study (NCCTG Trial, N01C5). J. Support Oncol. 2011, 9, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.M.; Potterat, O.; Seibert, I.; Fertig, O.; Meyer Zu Schwabedissen, H.E. Hyperforin-Induced Activation of the Pregnane X Receptor Is Influenced by the Organic Anion-Transporting Polypeptide 2B1. Mol. Pharm. 2019, 95, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.H.; Zheng, Y. A meta-analysis on the efficacy and safety of St John’s wort extract in depression therapy in comparison with selective serotonin reuptake inhibitors in adults. Neuropsychiatr. Dis. Treat 2016, 12, 1715–1723. [Google Scholar]
- Basar, S. Phytochemical Investigations on Boswellia Species; Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky March 2005. Available online: https://ediss.sub.uni-hamburg.de/2005 (accessed on 1 April 2022).
- Seitz, S. Isolierung und Strukturaufklärung von Entzündungshemmenden Inhaltsstoffen aus Weihrauchharz. 2008. Available online: https://publikationen.sulb.uni-saarland.de2008 (accessed on 1 April 2022).
- Ammon, H.P.T. Boswellic extracts and 11-keto-ss-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines. Phytomedicine 2019, 63, 153002. [Google Scholar] [CrossRef] [PubMed]
- Memorial Sloan Kettering Cancer Center about Herbs, Botanicals & Other Products. Available online: https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs (accessed on 7 February 2021).
- Springer. Federal Office of Consumer Protectionand Food Safety: List of Substances of the Competent Federal Government and Federal State Authorities: Category "Plants and Plant Parts" (Stoffliste des Bundes und der Bundesländer: Kategorie „Pflanzen und Pflanzenteile“), 1st ed.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Efferth, T.; Oesch, F. Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin. Cancer Biol. 2022, 80, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Winter, S.; Groscurth, P.; Safayhi, H.; Sailer, E.R.; Ammon, H.P.; Schabet, M.; Weller, M. Boswellic acids and malignant glioma: Induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer 1999, 80, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winking, M.; Sarikaya, S.; Rahmanian, A.; Jödicke, A.; Böker, D.K. Boswellic acids inhibit glioma growth: A new treatment option? J. Neurooncol. 2000, 46, 97–103. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Estrada, A.C.; Syrovets, T.; Pitterle, K.; Lunov, O.; Büchele, B.; Schimana-Pfeifer, J.; Schmidt, T.; Morad, S.A.; Simmet, T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol. Pharm. 2010, 77, 378–387. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, J.H.; Bondar, J.; Harwalkar, J.A.; Safayhi, H.; Golubic, M. Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells. Planta Med. 2002, 68, 397–401. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Gudjonsson, T.; Nielsen, O.H.; Vainer, B.; Seidelin, J.B. Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 395–404. [Google Scholar] [CrossRef]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [Green Version]
- Akincilar, S.C.; Khattar, E.; Boon, P.L.; Unal, B.; Fullwood, M.J.; Tergaonkar, V. Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov. 2016, 6, 1276–1291. [Google Scholar] [CrossRef] [Green Version]
- Khattar, E.; Kumar, P.; Liu, C.Y.; Akıncılar, S.C.; Raju, A.; Lakshmanan, M.; Maury, J.J.P.; Qiang, Y.; Li, S.; Tan, E.Y.; et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J. Clin. Investig. 2016, 126, 4045–4060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Padmavathi, G.; Kotoky, J.; Golla, R.; Kunnumakkara, A.B. Specific Targeting of Akt Kinase Isoforms: Taking the Precise Path for Prevention and Treatment of Cancer. Curr. Drug Targets 2017, 18, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Lou, W.; Fung, K.M.; Wolley, C.L.; Suhail, M.M.; Lin, H.K. Cancer Chemopreventive Effects of Boswellia sacra Gum Resin Hydrodistillates on Invasive Urothelial Cell Carcinoma: Report of a Case. Integr. Cancer 2017, 16, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnaraju, A.V.; Sundararaju, D.; Vamsikrishna, U.; Suryachandra, R.; Machiraju, G.; Sengupta, K.; Trimurtulu, G. Safety and toxicological evaluation of Aflapin: A novel Boswellia-derived anti-inflammatory product. Toxicol. Mech. Methods 2010, 20, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Kirste, S.; Treier, M.; Wehrle, S.J.; Becker, G.; Abdel-Tawab, M.; Gerbeth, K.; Hug, M.J.; Lubrich, B.; Grosu, A.L.; Momm, F. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer 2011, 117, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Lalithakumari, K.; Krishnaraju, A.V.; Sengupta, K.; Subbaraju, G.V.; Chatterjee, A. Safety and Toxicological Evaluation of a Novel, Standardized 3-O-Acetyl-11-keto-beta-Boswellic Acid (AKBA)-Enriched Boswellia serrata Extract (5-Loxin(R)). Toxicol. Mech. Methods 2006, 16, 199–226. [Google Scholar] [CrossRef]
- Togni, S.; Maramaldi, G.; Bonetta, A.; Giacomelli, L.; Di Pierro, F. Clinical evaluation of safety and efficacy of Boswellia-based cream for prevention of adjuvant radiotherapy skin damage in mammary carcinoma: A randomized placebo controlled trial. Eur. Rev. Med. Pharm. Sci. 2015, 19, 1338–1344. [Google Scholar]
- Dobat, K.; Dressendorfer, W. Leonhart Fuchs: The New Herbal of 1543; Taschen: Köln, Germany, 2001. [Google Scholar]
- Schad, F.; Thronicke, A.; Merkle, A.; Steele, M.L.; Kröz, M.; Herbstreit, C.; Matthes, H. Implementation of an Integrative Oncological Concept in the Daily Care of a German Certified Breast Cancer Center. Complement. Med. Res. 2018, 25, 85–91. [Google Scholar] [CrossRef]
- Drozdoff, L.; Klein, E.; Kiechle, M.; Paepke, D. Use of biologically-based complementary medicine in breast and gynecological cancer patients during systemic therapy. BMC Complement. Altern. Med. 2018, 18, 259. [Google Scholar] [CrossRef]
- Steele, M.L.; Axtner, J.; Happe, A.; Kröz, M.; Matthes, H.; Schad, F. Adverse Drug Reactions and Expected Effects to Therapy with Subcutaneous Mistletoe Extracts (Viscum album L.) in Cancer Patients. Evid. Based Complement Altern. Med. 2014, 2014, 724258. [Google Scholar] [CrossRef] [Green Version]
- Urech, K.; Jaggy, C.; Schaller, G. 12. Viscotoxin and mistletoe lectin contents in Viscum album L.—pharmaceutical implications. Phytomedicine: Int. J. Phytother. Phytopharm. 2007, 14, S16. [Google Scholar]
- Schaller, G.; Urech, K.; Giannattasio, M. Cytotoxicity of Different Viscotoxins and Extracts from the European Subspecies of Viscum album L. Phytother. Res. 1996, 10, 473–477. [Google Scholar] [CrossRef]
- Schaller, G.; Urech, K.; Grazi, G.; Giannattasio, M. Viscotoxin Composition of the three European Subspecies of Viscum album. Planta Med. 1998, 64, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Kleinsimon, S.; Kauczor, G.; Jaeger, S.; Eggert, A.; Seifert, G.; Delebinski, C. ViscumTT induces apoptosis and alters IAP expression in osteosarcoma in vitro and has synergistic action when combined with different chemotherapeutic drugs. BMC Complementary Altern. Med. 2017, 17, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, R.; Kim, M.S.; So, H.S.; Jung, B.H.; Moon, S.R.; Chung, S.Y.; Ko, C.B.; Kim, B.R.; Chung, H.T. Activation of c-Jun N-terminal kinase 1 (JNK1) in mistletoe lectin II-induced apoptosis of human myeloleukemic U937 cells. Biochem. Pharm. 2000, 60, 1685–1691. [Google Scholar] [CrossRef]
- Elluru, S.; Duong Van Huyen, J.P.; Delignat, S.; Prost, F.; Bayry, J.; Kazatchkine, M.D.; Kaveri, S.V. Molecular mechanisms underlying the immunomodulatory effects of mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung 2006, 56, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Elluru, S.R.; Duong Van Huyen, J.-P.; Wootla, B.; Delignat, S.; Prost, F.; Negi, V.; Kaveri, S. Tumor regressive effects of Viscum album preparations. Exploration of immunomodulatory mechanisms. Medicina 2007, 67, 85–89. [Google Scholar]
- Büssing, A.; Scheer, R.; Bauer, R.; Becker, H.; Berg, A.P.; Fintelmann, V. Viscum album L.—Mechanismen der Zytotoxizität. Die Mistel in der Tumortherapie; KVC Verlag: Essen, Germany, 2001. [Google Scholar]
- Klein, R.; Scheer, R.; Alban, S.; Becker, H. Effekte von Mistelextrakten auf immunkompetente Zellen in vitro und in vivo. In Die Mistel in der Tumortherapie 2; KVC Verlag: Essen, Germany, 2009. [Google Scholar]
- Kienle, G.S.; Kiene, H. Verträglichkeit, Nebenwirkungen, Überempfindlichkeitsreaktionen, Toxizität. In Die Mistel in der Onkologie. Fakten und Konzeptionelle Grundlagen; Schattauer Verlag: Stuttgart, Germany, 2003; pp. 591–607. [Google Scholar]
- Huber, R.; Classen, K.; Werner, M.; Klein, R. In vitro immunoreactivity towards lectin-rich or viscotoxin-rich mistletoe (Viscum album L.) extracts Iscador applied to healthy individuals. Arzneimittelforschung 2006, 56, 447–456. [Google Scholar] [CrossRef]
- Kienle, G.S.; Kiene, H. Die Mistel in der Onkologie: Fakten und konzeptionelle Grundlagen; Schattauer Verlag: Stuttgart, Germany, 2003. [Google Scholar]
- Elsässer-Beile, U.; Voss, M.; Schühle, R.; Wetterauer, U. Biological effects of natural and recombinant mistletoe lectin and an aqueous mistletoe extract on human monocytes and lymphocytes in vitro. J. Clin. Lab. Anal. 2000, 14, 255–259. [Google Scholar] [CrossRef]
- Tabiasco, J.; Pont, F.; Fournié, J.J.; Vercellone, A. Mistletoe viscotoxins increase natural killer cell-mediated cytotoxicity. Eur. J. Biochem. 2002, 269, 2591–2600. [Google Scholar] [CrossRef]
- Fischer, A. Charakterisierung der Immunmodulatorischen Wirkung von Mistelpräparaten auf Zellen des Immunsystems bei Rindern. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hannover, Germany, November 2006. [Google Scholar]
- Steinborn, C.; Klemd, A.M.; Sanchez-Campillo, A.S.; Rieger, S.; Scheffen, M.; Sauer, B.; Garcia-Käufer, M.; Urech, K.; Follo, M.; Ücker, A.; et al. Viscum album neutralizes tumor-induced immunosuppression in a human in vitro cell model. PLoS ONE 2017, 12, e0181553. [Google Scholar] [CrossRef] [PubMed]
- Saha, C.; Das, M.; Stephen-Victor, E.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Differential Effects of Viscum album Preparations on the Maturation and Activation of Human Dendritic Cells and CD4⁺ T Cell Responses. Molecules 2016, 21, 912. [Google Scholar] [CrossRef] [Green Version]
- Grossarth-Maticek, R.; Kiene, H.; Baumgartner, S.M.; Ziegler, R. Use of Iscador, an extract of European mistletoe (Viscum album), in cancer treatment: Prospective nonrandomized and randomized matched-pair studies nested within a cohort study. Altern. Health Med. 2001, 7, 57–66, 68–72, 74–76. [Google Scholar]
- Grossarth-Maticek, R.; Ziegler, R. Prospective controlled cohort studies on long-term therapy of ovairian cancer patients with mistletoe (Viscum album L.) extracts iscador. Arzneimittelforschung 2007, 57, 665–678. [Google Scholar] [PubMed] [Green Version]
- Grossarth-Maticek, R.; Ziegler, R. Randomized and non-randomized prospective controlled cohort studies in matched pair design for the long-term therapy of corpus uteri cancer patients with a mistletoe preparation (Iscador). Eur. J. Med. Res. 2008, 13, 107–120. [Google Scholar]
- Cazacu, M.; Oniu, T.; Lungoci, C.; Mihailov, A.; Cipak, A.; Klinger, R.; Weiss, T.; Zarkovic, N. The influence of isorel on the advanced colorectal cancer. Cancer Biother. Radiopharm. 2003, 18, 27–34. [Google Scholar] [CrossRef]
- Lenartz, D.; Dott, U.; Menzel, J.; Schierholz, J.M.; Beuth, J. Survival of glioma patients after complementary treatment with galactoside-specific lectin from mistletoe. Anticancer Res. 2000, 20, 2073–2076. [Google Scholar]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur. J. Cancer 2013, 49, 3788–3797. [Google Scholar] [CrossRef] [Green Version]
- Dold, U.; Edler, L.; Mäurer, H.-C. Krebszusatztherapie beim fortgeschrittenen nicht-kleinzelligen Bronchialkarzinom. Dtsch. Arztebl. Int. 1992, 89, A-3797. [Google Scholar]
- Salzer, G.; Danmayr, E.; Wutzlhofer, F.; Frey, S. Adjuvante Iscador-Behandlung operierter nicht kleinzelliger Bronchuskarzinome. Dtsch. Z. Onkol. 1991, 23, 93–98. [Google Scholar]
- Kleeberg, U.R.; Suciu, S.; Bröcker, E.B.; Ruiter, D.J.; Chartier, C.; Liénard, D.; Marsden, J.; Schadendorf, D.; Eggermont, A.M. Final results of the EORTC 18871/DKG 80-1 randomised phase III trial. rIFN-alpha2b versus rIFN-gamma versus ISCADOR M versus observation after surgery in melanoma patients with either high-risk primary (thickness >3 mm) or regional lymph node metastasis. Eur. J. Cancer 2004, 40, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Grossarth-Maticek, R.; Ziegler, R. Prospective controlled cohort studies on long-term therapy of breast cancer patients with a mistletoe preparation (Iscador). Komplementmed 2006, 13, 285–292. [Google Scholar] [CrossRef]
- Grossarth-Maticek, R.; Ziegler, R. Efficacy and safety of the long-term treatment of melanoma with a mistletoe preparation (Iscador). Schweiz. Z. Ganzheitsmed. 2007, 19, 325–332. [Google Scholar] [CrossRef]
- Grossarth-Maticek, R.; Ziegler, R. Prospective controlled cohort studies on long-term therapy of cervical cancer patients with a mistletoe preparation (Iscador). Komplementmed 2007, 14, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Wollner, M.; Hammer, L.; Agbarya, A.; Dudnik, E.; Haim, N. Mistletoe as complementary treatment in patients with advanced non-small-cell lung cancer treated with carboplatin-based combinations: A randomised phase II study. Eur. J. Cancer 2013, 49, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Steuer-Vogt, M.K.; Bonkowsky, V.; Ambrosch, P.; Scholz, M.; Neiss, A.; Strutz, J.; Hennig, M.; Lenarz, T.; Arnold, W. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: A randomised controlled clinical trial. Eur. J. Cancer 2001, 37, 23–31. [Google Scholar] [CrossRef]
- Goebell, P.; Otto, T.; Suhr, J.; Rübben, H. Evaluation of an Unconventional Treatment Modality with Mistletoe Lectin to Prevent Recurrence of Superficial Bladder Cancer: A Randomized Phase ii Trial. J. Urol. 2002, 168, 72–75. [Google Scholar] [CrossRef]
- Borrelli, E. Evaluation of the quality of life in breast cancer patients undergoing lectin standardized mistletoe therapy. Minerva Med. 2001, 92, 105–107. [Google Scholar]
- Semiglasov, V.F.; Stepula, V.V.; Dudov, A.; Lehmacher, W.; Mengs, U. The standardised mistletoe extract PS76A2 improves QoL in patients with breast cancer receiving adjuvant CMF chemotherapy: A randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2004, 24, 1293–1302. [Google Scholar]
- Semiglazov, V.F.; Stepula, V.V.; Dudov, A.; Schnitker, J.; Mengs, U. Quality of life is improved in breast cancer patients by Standardised Mistletoe Extract PS76A2 during chemotherapy and follow-up: A randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006, 26, 1519–1529. [Google Scholar]
- Piao, B.K.; Wang, Y.X.; Xie, G.R.; Mansmann, U.; Matthes, H.; Beuth, J.; Lin, H.S. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004, 24, 303–309. [Google Scholar] [PubMed]
- Mei, N.; Guo, X.; Ren, Z.; Kobayashi, D.; Wada, K.; Guo, L. Review of Ginkgo biloba-induced toxicity, from experimental studies to human case reports. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2017, 35, 1–28. [Google Scholar] [CrossRef] [PubMed]
- DeFeudis, F.V.; Papadopoulos, V.; Drieu, K. Ginkgo biloba extracts and cancer: A research area in its infancy. Fundam. Clin. Pharm. 2003, 17, 405–417. [Google Scholar] [CrossRef]
- Sagar, S.M.; Yance, D.; Wong, R.K. Natural health products that inhibit angiogenesis: A potential source for investigational new agents to treat cancer-Part 1. Curr. Oncol. 2006, 13, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zheng, J.; Jin, X.; Wei, G.; Wang, G.; Sun, X.; Li, X. Ginkgolic acid inhibits the invasiveness of colon cancer cells through AMPK activation. Oncol. Lett. 2017, 14, 5831–5838. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.-R.; Liu, P.-L.; Chen, Y.-H.; Chou, S.-H.; Yang, M.-C.; Cheng, Y.-J.; Hwang, J.-J.; Yin, W.-H.; Chong, I.-W. Ginkgo biloba Extract Decreases Non-Small Cell Lung Cancer Cell Migration by Downregulating Metastasis-Associated Factor Heat-Shock Protein 27. PLoS ONE 2014, 9, e91331. [Google Scholar] [CrossRef]
- Han, D.; Cao, C.; Su, Y.; Wang, J.; Sun, J.; Chen, H.; Xu, A. Ginkgo biloba exocarp extracts inhibits angiogenesis and its effects on Wnt/β-catenin-VEGF signaling pathway in Lewis lung cancer. J. Ethnopharmacol. 2016, 192, 406–412. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Cheng, Y.; Du, J.; Chen, D.; Li, C.; Zhang, J. Apoptosis Induced by Ginkgo biloba (EGb761) in Melanoma Cells Is Mcl-1-Dependent. PLoS ONE 2015, 10, e0124812. [Google Scholar] [CrossRef]
- Kim, K.S.; Rhee, K.H.; Yoon, J.H.; Lee, J.G.; Lee, J.H.; Yoo, J.B. Ginkgo biloba extract (EGb 761) induces apoptosis by the activation of caspase-3 in oral cavity cancer cells. Oral Oncol. 2005, 41, 383–389. [Google Scholar] [CrossRef]
- Czauderna, C.; Palestino-Dominguez, M.; Castven, D.; Becker, D.; Zanon-Rodriguez, L.; Hajduk, J.; Mahn, F.L.; Herr, M.; Strand, D.; Strand, S.; et al. Ginkgo biloba induces different gene expression signatures and oncogenic pathways in malignant and non-malignant cells of the liver. PLoS ONE 2018, 13, e0209067. [Google Scholar] [CrossRef]
- Fu, Z.; Lin, L.; Liu, S.; Qin, M.; He, S.; Zhu, L.; Huang, J. Ginkgo Biloba Extract Inhibits Metastasis and ERK/Nuclear Factor kappa B (NF-κB) Signaling Pathway in Gastric Cancer. Med. Sci. Monit. 2019, 25, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-R.; Yang, H. Ginkgolic acid (GA) suppresses gastric cancer growth by inducing apoptosis and suppressing STAT3/JAK2 signaling regulated by ROS. Biomed. Pharmacother. 2020, 125, 109585. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kim, J.H.; Song, K.; Kim, S.H.; Yoon, J.H.; Kim, K.S. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother. Res. 2010, 24 (Suppl. 1), S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Weakley, S.M.; Wang, X.; Mu, H.; Lü, J.; Lin, P.H.; Yao, Q.; Chen, C. Ginkgolide A-gold nanoparticles inhibit vascular smooth muscle proliferation and migration in vitro and reduce neointimal hyperplasia in a mouse model. J. Surg. Res. 2011, 171, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Shao, Q.H.; Zhou, H.; Wu, J.L.; Quan, W.Q.; Ji, P.; Yao, Y.W.; Li, D.; Sun, Z.J. Ginkgolide B inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement. Med. 2020, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Pan, J.; Shen, W.; He, P.; Zheng, J.; Zhou, X.; Lu, G.; Chen, Z.; Zhou, Z. Ginkgolide B Inhibits Human Bladder Cancer Cell Migration and Invasion through MicroRNA-223-3p. Cell Physiol. Biochem. 2016, 39, 1787–1794. [Google Scholar] [CrossRef]
- Yang, M.H.; Ha, I.J.; Lee, S.G.; Lee, J.; Um, J.Y.; Ahn, K.S. Ginkgolide C promotes apoptosis and abrogates metastasis of colorectal carcinoma cells by targeting Wnt/β-catenin signaling pathway. IUBMB Life 2021, 73, 1222–1234. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Zhang, Y.; Alharbi, S.A.; Shi, Y. Sesquiterpenoid bilobalide inhibits gastric carcinoma cell growth and induces apoptosis both in vitro and in vivo models. J. Biochem. Mol. Toxicol. 2021, 35, e22723. [Google Scholar] [CrossRef]
- Xu, A.H.; Chen, H.S.; Sun, B.C.; Xiang, X.R.; Chu, Y.F.; Zhai, F.; Jia, L.C. Therapeutic mechanism of ginkgo biloba exocarp polysaccharides on gastric cancer. World J. Gastroenterol. 2003, 9, 2424–2427. [Google Scholar] [CrossRef]
- Ye, B.; Aponte, M.; Dai, Y.; Li, L.; Ho, M.C.; Vitonis, A.; Edwards, D.; Huang, T.N.; Cramer, D.W. Ginkgo biloba and ovarian cancer prevention: Epidemiological and biological evidence. Cancer Lett. 2007, 251, 43–52. [Google Scholar] [CrossRef]
- Biggs, M.L.; Sorkin, B.C.; Nahin, R.L.; Kuller, L.H.; Fitzpatrick, A.L. Ginkgo biloba and risk of cancer: Secondary analysis of the Ginkgo Evaluation of Memory (GEM) Study. Pharm. Drug Saf. 2010, 19, 694–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, D.L.; Burger, K.; Novotny, P.J.; Fitch, T.R.; Kohli, S.; Soori, G.; Wilwerding, M.B.; Sloan, J.A.; Kottschade, L.A.; Rowland, K.M., Jr.; et al. The use of Ginkgo biloba for the prevention of chemotherapy-related cognitive dysfunction in women receiving adjuvant treatment for breast cancer, N00C9. Support Care Cancer 2013, 21, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: Considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine 2005, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Grosse, Y.; Loomis, D.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of some drugs and herbal products. Lancet Oncol. 2013, 14, 807–808. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer IARC Monographs on the Identification of Carcinogenic Hazards to Human. Available online: https://monographs.iarc.who.int (accessed on 1 April 2022).
- Choi, E.J.; Bae, S.M.; Ahn, W.S. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch. Pharm. Res. 2008, 31, 1281–1285. [Google Scholar] [CrossRef]
- Unlu, A.; Nayir, E.; Kirca, O.; Ay, H.; Ozdogan, M. Ginseng and cancer. J. Buon 2016, 21, 1383–1387. [Google Scholar]
- Mochizuki, M.; Yoo, Y.C.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tono-oka, S.; Samukawa, K.; Azuma, I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Z.; Anderson, S.; Du, W.; He, T.C.; Yuan, C.S. Red ginseng and cancer treatment. Chin. J. Nat. Med. 2016, 14, 7–16. [Google Scholar]
- King, M.L.; Murphy, L.L. American ginseng (Panax quinquefolius L.) extract alters mitogen-activated protein kinase cell signaling and inhibits proliferation of MCF-7 cells. J. Exp. Oncol. 2007, 6, 147–155. [Google Scholar]
- Li, B.; Wang, C.Z.; He, T.C.; Yuan, C.S.; Du, W. Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2010, 289, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Hofseth, A.B.; Cui, X.; Windust, A.J.; Poudyal, D.; Chumanevich, A.A.; Matesic, L.E.; Singh, N.P.; Nagarkatti, M.; Nagarkatti, P.S.; et al. American Ginseng Suppresses Colitis through p53-Mediated Apoptosis of Inflammatory Cells. Cancer Prev. Res. 2010, 3, 339–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, A.; Hao, H.; Zheng, X.; Liang, Y.; Xie, Y.; Xie, T.; Dai, C.; Zhao, Q.; Wu, X.; Xie, L.; et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J. Neuroinflamm. 2011, 8, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kwon, M.C.; Jang, J.P.; Sohng, J.K.; Jung, H.J. The ginsenoside metabolite compound K inhibits growth, migration and stemness of glioblastoma cells. Int. J. Oncol. 2017, 51, 414–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharm. 2009, 7, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.; Kim, J.H.; Kim, J.H.; Yi, Y.S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Xing, X.; Qiao, J.; Yin, Y.; Wang, J.; Liu, M.; Zhang, W. Ginsenoside Rh2 stimulates the production of mitochondrial reactive oxygen species and induces apoptosis of cervical cancer cells by inhibiting mitochondrial electron transfer chain complex. Mol. Med. Rep. 2021, 24, 873. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.S.; Zhang, E.T.; Li, G.A.; Liu, W.Y.; Li, Y.; Jin, Y.H. (20S) Ginsenoside Rh2 Exerts Its Anti-Tumor Effect by Disrupting the HSP90A-Cdc37 System in Human Liver Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13170. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.W.; Yun, S.H.; Kim, S.J. Ginsenoside Rh2 upregulates long noncoding RNA STXBP5-AS1 to sponge microRNA-4425 in suppressing breast cancer cell proliferation. J. Ginseng Res. 2021, 45, 754–762. [Google Scholar] [CrossRef]
- Jeon, H.; Huynh, D.T.N.; Baek, N.; Nguyen, T.L.L.; Heo, K.S. Ginsenoside-Rg2 affects cell growth via regulating ROS-mediated AMPK activation and cell cycle in MCF-7 cells. Phytomedicine 2021, 85, 153549. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, P.; Li, H.; Liu, Y.; Wang, T.; Cheng, Y. Ginsenoside Rh2 Inhibits Glycolysis through the STAT3/c-MYC Axis in Non-Small-Cell Lung Cancer. J. Oncol. 2021, 2021, 9715154. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.N.; Vexler, A.; Kalich-Philosoph, L.; Yoo, H.S.; Kwon, K.R.; Yadgar, M.; Bondar, E.; Bar-Shai, A.; Volovitz, I.; et al. Rh2-enriched Korean ginseng (Ginseng Rh2+) inhibits tumor growth and development of metastasis of non-small cell lung cancer. Food Funct. 2021, 12, 8068–8077. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.K.; Jeong, Y.J.; Cho, H.J.; Park, Y.Y.; Song, K.H.; Chang, Y.C. Rg3-enriched red ginseng extract promotes lung cancer cell apoptosis and mitophagy by ROS production. J. Ginseng Res. 2022, 46, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lv, Q.; Li, Y.; Jin, Y.H. The Anti-Tumor Effect and Underlying Apoptotic Mechanism of Ginsenoside Rk1 and Rg5 in Human Liver Cancer Cells. Molecules 2021, 26, 3926. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Zou, Z.; Pan, Q.; Li, X.; Liang, Z.; Li, L.; Lin, Y.; Peng, X.; Zhang, R.; et al. Ginsenoside (20S)-protopanaxatriol induces non-protective autophagy and apoptosis by inhibiting Akt/mTOR signaling pathway in triple-negative breast cancer cells. Biochem. Biophys. Res. Commun. 2021, 583, 184–191. [Google Scholar] [CrossRef]
- Cui, Y.; Shu, X.O.; Gao, Y.T.; Cai, H.; Tao, M.H.; Zheng, W. Association of ginseng use with survival and quality of life among breast cancer patients. Am. J. Epidemiol. 2006, 163, 645–653. [Google Scholar] [CrossRef]
- Donovan, K.A.; Jacobsen, P.B. The Fatigue Symptom Inventory: A systematic review of its psychometric properties. Support Care Cancer 2010, 19, 169–185. [Google Scholar] [CrossRef]
- Jiang, S.L.; Liu, H.J.; Liu, Z.C.; Liu, N.; Liu, R.; Kang, Y.R.; Ji, J.G.; Zhang, C.; Hua, B.J.; Kang, S.J. Adjuvant effects of fermented red ginseng extract on advanced non-small cell lung cancer patients treated with chemotherapy. Chin. J. Integr. Med. 2017, 23, 331–337. [Google Scholar] [CrossRef]
- Barton, D.L.; Soori, G.S.; Bauer, B.A.; Sloan, J.A.; Johnson, P.A.; Figueras, C.; Duane, S.; Mattar, B.; Liu, H.; Atherton, P.J.; et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: A randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer 2010, 18, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Shuman-Paretsky, M.J.; Belser-Ehrlich, J.; Holtzer, R. Psychometric properties of the Brief Fatigue Inventory in community-dwelling older adults. Arch. Phys. Med. Rehabil. 2014, 95, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2021, 69, 140–149. [Google Scholar] [CrossRef]
- European Medicines Agency Community Herbal Monograph on Zingiber Officinale Roscoe, Rhizoma. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-zingiber-officinale-roscoe-rhizoma_en.pdf (accessed on 1 April 2022).
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-W.; Oh, D.-H.; Jung, C.; Kwon, D.-D.; Lim, Y.-C. Apoptotic Effects of 6-Gingerol in LNCaP Human Prostate Cancer Cells. Soonchunhyang Med. Sci. 2011, 17, 75–79. [Google Scholar] [CrossRef]
- Hung, J.Y.; Hsu, Y.L.; Li, C.T.; Ko, Y.C.; Ni, W.C.; Huang, M.S.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem. 2009, 57, 9809–9816. [Google Scholar] [CrossRef]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-κB activation. Br. J. Pharm. 2010, 161, 1763–1777. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.M.; Yao, X.H.; Hao, Y.H.; Pan, A.H.; Zhou, X.W. 8-Gingerol regulates colorectal cancer cell proliferation and migration through the EGFR/STAT/ERK pathway. Int. J. Oncol. 2020, 56, 390–397. [Google Scholar] [CrossRef]
- Ryu, M.J.; Chung, H.S. [10]-Gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells. Vitr. Cell Dev. Biol. Anim. 2015, 51, 92–101. [Google Scholar] [CrossRef]
- Fuzer, A.M.; Martin, A.; Becceneri, A.B.; da Silva, J.A.; Vieira, P.C.; Cominetti, M.R. [10]-Gingerol Affects Multiple Metastatic Processes and Induces Apoptosis in MDAMB- 231 Breast Tumor Cells. Anticancer Agents Med. Chem. 2019, 19, 645–654. [Google Scholar] [CrossRef]
- Martin, A.; Fuzer, A.M.; Becceneri, A.B.; da Silva, J.A.; Tomasin, R.; Denoyer, D.; Kim, S.H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B.; et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget 2017, 8, 72260–72271. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, A.; Murphy, K.; Hoskin, D.W. 10-Gingerol Inhibits Ovarian Cancer Cell Growth by Inducing G2 Arrest. Adv. Pharm. Bull. 2019, 9, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Chen, H.; Soroka, D.N.; Warin, R.F.; Sang, S. Cysteine-Conjugated Metabolites of Ginger Components, Shogaols, Induce Apoptosis through Oxidative Stress-Mediated p53 Pathway in Human Colon Cancer Cells. J. Agric. Food Chem. 2014, 62, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Kaewtunjai, N.; Wongpoomchai, R.; Imsumran, A.; Pompimon, W.; Athipornchai, A.; Suksamrarn, A.; Lee, T.R.; Tuntiwechapikul, W. Ginger Extract Promotes Telomere Shortening and Cellular Senescence in A549 Lung Cancer Cells. ACS Omega 2018, 3, 18572–18581. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.Y.; Choi, J.S.; Kim, J.E.; Park, C.; Jeong, J.W. Zingerone suppresses angiogenesis via inhibition of matrix metalloproteinases during tumor development. Oncotarget 2016, 7, 47232–47241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Weng, C.J.; Wu, C.F.; Huang, H.W.; Ho, C.T.; Yen, G.C. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol. Nutr. Food Res. 2010, 54, 1618–1627. [Google Scholar] [CrossRef]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Tao, Y.; Li, W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 2011, 82, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.F.; Umar, A.; Hawk, E.T. Cyclooxygenase inhibition in cancer prevention and treatment. Expert Opin. Pharm. 2003, 4, 2193–2204. [Google Scholar] [CrossRef]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. 2013, 6, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Shokri, F.; Mostafa Gharebaghi, P.; Esfahani, A.; Sayyah-Melli, M.; Jafari Shobeiri, M.; Ouladsahebmadarek, E.; Ghojazadeh, M. Comparison of the Complications of Platinum-Based Adjuvant Chemotherapy With and Without Ginger in a Pilot Study on Ovarian Cancer Patients. Int. J. Women’s Health Reprod. Sci. 2016, 5, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Oh, H. Ginger as an antiemetic modality for chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis. Oncol. Nurs. Forum 2013, 40, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McCarthy, A.L.; Ried, K.; McKavanagh, D.; Vitetta, L.; Sali, A.; Lohning, A.; Isenring, E. The Effect of a Standardized Ginger Extract on Chemotherapy-Induced Nausea-Related Quality of Life in Patients Undergoing Moderately or Highly Emetogenic Chemotherapy: A Double Blind, Randomized, Placebo Controlled Trial. Nutrients 2017, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, A. Turmeric (curcumin) in biliary diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.-L.; Huang, T.-S.; Lin, J.-K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1996, 1317, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, Y.M.; Yu, Y.; Cao, C.S.; Zhang, J.H.; Li, K.; Zhang, P.Y. Curcumin and resveratrol in combination modulate drug-metabolizing enzymes as well as antioxidant indices during lung carcinogenesis in mice. Hum. Exp. Toxicol. 2015, 34, 620–627. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef] [Green Version]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, Y.M.; Zhang, P.Y. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharm. Sci. 2015, 19, 1736–1743. [Google Scholar]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gao, Y.; Li, L.; Xie, S. Curcumin Inhibits Papillary Thyroid Cancer Cell Proliferation by Regulating lncRNA LINC00691. Anal. Cell Pathol. 2022, 2022, 5946670. [Google Scholar] [CrossRef] [PubMed]
- Guneydas, G.; Topcul, M.R. Antiproliferative Effects of Curcumin Different Types of Breast Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 911–917. [Google Scholar] [CrossRef]
- Yavuz Türel, G.; Şahin Calapoğlu, N.; Bayram, D.; Özgöçmen, M.; Toğay, V.A.; Evgen Tülüceoğlu, E. Curcumin induces apoptosis through caspase dependent pathway in human colon carcinoma cells. Mol. Biol. Rep. 2022, 49, 1351–1360. [Google Scholar] [CrossRef]
- Trošelj, K.G.; Samaržija, I.; Tomljanović, M.; Kujundžić, R.N.; Đaković, N.; Mojzeš, A. Implementing Curcumin in Translational Oncology Research. Molecules 2020, 25, 5240. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A., Jr.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Mansourian, A.; Amanlou, M.; Shirazian, S.; Moosavian Jahromi, Z.; Amirian, A. The effect of “Curcuma Longa” topical gel on radiation-induced oral mucositis in patients with head and neck cancer. Int. J. Radiat. Res. 2015, 13, 269–274. [Google Scholar]
- Rao, S.; Dinkar, C.; Vaishnav, L.K.; Rao, P.; Rai, M.P.; Fayad, R.; Baliga, M.S. The Indian Spice Turmeric Delays and Mitigates Radiation-Induced Oral Mucositis in Patients Undergoing Treatment for Head and Neck Cancer: An Investigational Study. Integr. Cancer 2014, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.L.; Wilcox, R.; Price, J.M.; Li, L.; Dai, H.; Freeman, K.M.; Friley, W.W.; Herman, A.G.; Black, C.B.; Brouwer, K.R.; et al. An Evaluation of Potential Inhibition of CYP3A4/5 and CYP2C9 Enzymatic Activity by Boswellia serrata Extract. Appl. Vitr. Toxicol. 2019, 5, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Schink, M.; Dehus, O. Effects of mistletoe products on pharmacokinetic drug turnover by inhibition and induction of cytochrome P450 activities. BMC Complement. Altern. Med. 2017, 17, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Fuse, E.; Figg, W.D. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 2002, 8, 1964–1973. [Google Scholar]
- Goey, A.K.; Mooiman, K.D.; Beijnen, J.H.; Schellens, J.H.; Meijerman, I. Relevance of in vitro and clinical data for predicting CYP3A4-mediated herb-drug interactions in cancer patients. Cancer Treat Rev. 2013, 39, 773–783. [Google Scholar] [CrossRef]
- European Medicines Agency Ginseng Radix. Available online: https://www.ema.europa.eu/en/medicines/herbal/ginseng-radix (accessed on 7 February 2021).
- Langhammer, A.J.; Nilsen, O.G. In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy. Phytother. Res. 2014, 28, 603–610. [Google Scholar] [CrossRef]
- Li, M.; Chen, P.Z.; Yue, Q.X.; Li, J.Q.; Chu, R.A.; Zhang, W.; Wang, H. Pungent ginger components modulates human cytochrome P450 enzymes in vitro. Acta Pharm. Sin. 2013, 34, 1237–1242. [Google Scholar] [CrossRef]
- Mukkavilli, R.; Gundala, S.R.; Yang, C.; Donthamsetty, S.; Cantuaria, G.; Jadhav, G.R.; Vangala, S.; Reid, M.D.; Aneja, R. Modulation of cytochrome P450 metabolism and transport across intestinal epithelial barrier by ginger biophenolics. PLoS ONE 2014, 9, e108386. [Google Scholar] [CrossRef]
- Shamsi, S.; Tran, H.; Tan, R.S.J.; Tan, Z.J.; Lim, L.Y. Curcumin, Piperine, and Capsaicin: A Comparative Study of Spice-Mediated Inhibition of Human Cytochrome P450 Isozyme Activities. Drug Metab. Dispos. 2017, 45, 49. [Google Scholar] [CrossRef] [Green Version]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Weber, C.C.; Reising, K.; Müller, W.E.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Modulation of Pgp function by boswellic acids. Planta Med. 2006, 72, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Hellum, B.H.; Nilsen, O.G. In vitro inhibition of CYP3A4 metabolism and P-glycoprotein-mediated transport by trade herbal products. Basic Clin. Pharm. Toxicol. 2008, 102, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-Y.; Kim, E.-H.; Kim, S.-W.; Kim, S.-N.; Park, J.-D.; Rhee, D.-K. Selective toxicity of ginsenoside Rg3 on multidrug resistant cells by membrane fluidity modulation. Arch. Pharmacal Res. 2008, 31, 171–177. [Google Scholar] [CrossRef]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Leechanachai, P.; Smith, M.M.; Ambudkar, S.V.; Limtrakul, P.-n. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem. Pharmacol. 2002, 64, 573–582. [Google Scholar] [CrossRef]
- Gu, C.; Qiao, J.; Zhu, M.; Du, J.; Shang, W.; Yin, W.; Wang, W.; Han, M.; Lu, W. Preliminary evaluation of the interactions of Panax ginseng and Salvia miltiorrhiza Bunge with 5-fluorouracil on pharmacokinetics in rats and pharmacodynamics in human cells. Am. J. Chin. Med. 2013, 41, 443–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsharmoghadam, N.; Haghighatian, Z.; Mazdak, H.; Mirkheshti, N.; Mehrabi Koushki, R.; Alavi, S.A. Concentration- Dependent Effects of Curcumin on 5-Fluorouracil Efficacy in Bladder Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18, 3225–3230. [Google Scholar]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Ferguson, J.E.; Orlando, R.A. Curcumin reduces cytotoxicity of 5-Fluorouracil treatment in human breast cancer cells. J. Med. Food 2015, 18, 497–502. [Google Scholar] [CrossRef]
- Jain, A.; Rani, V. Assessment of herb-drug synergy to combat doxorubicin induced cardiotoxicity. Life Sci. 2018, 205, 97–106. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Moon, S.H.; Cho, Y.S.; Choe, Y.S.; Lee, K.H. Effects of curcumin on cancer cell mitochondrial function and potential monitoring with ¹⁸F-FDG uptake. Oncol. Rep. 2016, 35, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moiseeva, E.P.; Almeida, G.M.; Jones, G.D.; Manson, M.M. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol. Cancer 2007, 6, 3071–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Seehofer, D.; Schirmeier, A.; Bengmark, S.; Carter, J.; Koch, M.; Glanemann, M.; Nüssler, A.K.; Neuhaus, P.; Menger, M.D. Inhibitory effect of curcumin on early liver regeneration following partial hepatectomy in rats. J. Surg. Res. 2009, 155, 195–200. [Google Scholar] [CrossRef]
- Somasundaram, S.; Edmund, N.A.; Moore, D.T.; Small, G.W.; Shi, Y.Y.; Orlowski, R.Z. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002, 62, 3868–3875. [Google Scholar]
- Yan, Y.D.; Kim, D.H.; Sung, J.H.; Yong, C.S.; Choi, H.G. Enhanced oral bioavailability of docetaxel in rats by four consecutive days of pre-treatment with curcumin. Int. J. Pharm. 2010, 399, 116–120. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Lu, N.; Yan, Z.; Wang, N. SAHA and curcumin combinations co-enhance histone acetylation in human cancer cells but operate antagonistically in exerting cytotoxic effects. J. Asian Nat. Prod. Res. 2010, 12, 335–348. [Google Scholar] [CrossRef]
- Rotblatt, M. Herbal Medicine: Expanded Commission E Monographs. Ann. Intern. Med. 2000, 133, 487. [Google Scholar] [CrossRef]
- Ammon, H.P. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. Adv. Exp. Med. Biol. 2016, 928, 291–327. [Google Scholar]
- Kienle, G.S.; Grugel, R.; Kiene, H. Safety of higher dosages of Viscum album L. in animals and humans—Systematic review of immune changes and safety parameters. BMC Complement. Altern. Med. 2011, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Ginseng. Drugs and Lactation Database (LactMed); National Library of Medicine (US): Bethesda, MD, USA, 2006.
- Roland, P.D.; Nergård, C.S. Ginkgo biloba—effect, adverse events and drug interaction. Tidsskr. Nor. Laegeforen. 2012, 132, 956–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules 2022, 27, 3209. https://doi.org/10.3390/molecules27103209
Zimmermann-Klemd AM, Reinhardt JK, Winker M, Gründemann C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules. 2022; 27(10):3209. https://doi.org/10.3390/molecules27103209
Chicago/Turabian StyleZimmermann-Klemd, Amy M., Jakob K. Reinhardt, Moritz Winker, and Carsten Gründemann. 2022. "Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options" Molecules 27, no. 10: 3209. https://doi.org/10.3390/molecules27103209