Enhanced Pharmaceutically Active Compounds Productivity from Streptomyces SUK 25: Optimization, Characterization, Mechanism and Techno-Economic Analysis
Abstract
:1. Introduction
2. Results
2.1. RSM-Based Optimization of PHACs Production
2.2. Validation of the Optimal Parameters
2.3. Column Chromatography and Thin-Layer Chromatography
2.4. Determination of Antibacterial Activity by Using MIC and Disc Diffusion Method
2.5. Fourier Transform Infrared Spectroscopy
2.6. GC-MS Analysis of Volatile Components
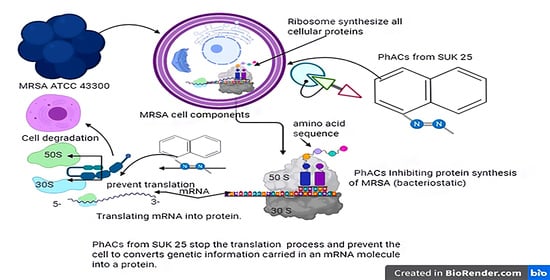
2.7. Gene Ontology and Pathway Analysis
2.8. Techno-Economic Analysis of the PhACs Productivity from SUK 25
2.9. Annual Operation Cost
2.10. PhACs Profitability and Annual Revenue
3. Discussion
4. Materials and Methods
4.1. Strain and Culture Condition
4.2. Basal Production Medium
4.3. RSM-Based Optimization of PHACs Production
4.4. Submerged Fermentation and Extraction of Secondary Metabolites Methods
4.5. Determination of Dependent Variables and Determination of Antibacterial Activity
4.6. Purification of PhACs by Column Chromatography and Thin-Layer Chromatography
4.7. Fourier Transform Infrared Spectroscopy Spectrometer
4.8. GC-MS Analysis of Volatile Components of Streptomyces SUK 25
4.9. Gene Ontology and Pathway Analysis
4.10. Techno-Economic Analysis of the PhACs Productivity from SUK 25
4.10.1. Process Simulation
4.10.2. Production and Extraction of PhACs in SmF Media
4.10.3. Column Chromatography and Thin-Layer Chromatography
4.10.4. Effectiveness of the PhACs and Determination of Antibacterial Activity
4.10.5. Economic Analysis
4.10.6. Annual Operation Cost
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Butini, M.E.; Abbandonato, G.; Di Rienzo, C.; Trampuz, A.; Di Luca, M. Isothermal Microcalorimetry Detects the Presence of Persister Cells in a Staphylococcus aureus Biofilm After Vancomycin Treatment. Front. Microbiol. 2019, 10, 332. [Google Scholar] [CrossRef]
- Qi, D.; Zou, L.; Zhou, D.; Chen, Y.; Gao, Z.; Feng, R.; Zhang, M.; Li, K.; Xie, J.; Wang, W. Taxonomy and Broad-Spectrum Antifungal Activity of Streptomyces sp. SCA3-4 Isolated from Rhizosphere Soil of Opuntia stricta. Front. Microbiol. 2019, 10, 1390. [Google Scholar] [CrossRef]
- Alshaibani, M.M.; Jalil, J.; Sidik, N.M.; Edrada-Ebel, R.; Zin, N.M. Isolation and characterization of cyclo-(tryptophanyl-prolyl) and chloramphenicol from Streptomyces sp. SUK 25 with antimethicillin-resistant Staphylococcus aureus activity. Drug Des. Devel. Ther. 2016, 10, 1817–1827. [Google Scholar]
- AlShaibani, M.M.; Jalil, J.; Sidik, N.M.; Ahmad, S.J.; Kamal, N.; Edrada-Ebel, R.; Zin, N.M. Isolation, purification and characterization of five active diketopiperazine derivatives from endophytic Streptomyces SUK 25 with antimicrobial and cytotoxic activities. J. Microbiol. Biotechnol. 2017, 27, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Pathak, L.; Singh, V.; Niwas, R.; Osama, K.; Khan, S.; Haque, S.; Tripathi, C.K.M.; Mishra, B.N. Artificial Intelligence versus Statistical Modeling and Optimization of Cholesterol Oxidase Production by using Streptomyces Sp. PLoS ONE 2015, 10, e0137268. [Google Scholar] [CrossRef] [Green Version]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Zin, N.M.; Al-Shaibani, M.M.; Jalil, J.; Sukri, A.; Al-Maleki, A.R.; Sidik, N.M. Profiling of gene expression in methicillin-resistant Staphylococcus aureus in response to cyclo-(l-Val-l-Pro) and chloramphenicol isolated from Streptomyces sp., SUK 25 reveals gene downregulation in multiple biological targets. Arch. Microbiol. 2020, 202, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Chen, J.; Zhang, X.; Guo, M.; Yu, G. A Literature Review of Gene Function Prediction by Modeling Gene Ontology. Front. Genet. 2020, 11, 400. [Google Scholar] [CrossRef]
- Mikhelkis, L.; Govindarajan, V. Techno-Economic and Partial Environmental Analysis of Carbon Capture and Storage (CCS) and Carbon Capture, Utilization, and Storage (CCU/S): Case Study from Proposed Waste-Fed District-Heating Incinerator in Sweden. Sustainability 2020, 12, 5922. [Google Scholar] [CrossRef]
- Kumar, V.; Al-Gheethi, A.; Asharuddin, S.M.; Othman, N. Potential of cassava peels as a sustainable coagulant aid for institutional wastewater treatment: Characterisation, optimisation and techno-economic analysis. Chem. Eng. J. 2020. [Google Scholar] [CrossRef]
- Teymouri, A.; Adams, K.J.; Dong, T.; Kumar, S. Evaluation of lipid extractability after flash hydrolysis of algae. Fuel 2018, 224, 23–31. [Google Scholar] [CrossRef]
- Kang, C.; Wen, T.-C.; Kang, J.-C.; Meng, Z.-B.; Li, G.-R.; Hyde, K.D. Optimization of Large-Scale Culture Conditions for the Production of Cordycepin with Cordyceps militarisby Liquid Static Culture. Sci. World J. 2014, 2014, 510627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, E.B.; Mazrad, Z.A.I.; Robby, A.I.; In, I.; Park, S.Y. Alkaline phosphatase-responsive fluorescent polymer probe coated surface for colorimetric bacteria detection. Eur. Polym. J. 2018, 105, 217–225. [Google Scholar] [CrossRef]
- Jayakumar, V.; Sundar, A.R.; Viswanathan, R. Biocontrol of Colletotrichum falcatum with volatile metabolites produced by endophytic bacteria and profiling VOCs by headspace SPME coupled with GC–MS. Sugar Tech. 2021, 23, 94–107. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Padmavathi, A.R.; Abinaya, B.; Pandian, S.K. Phenol, 2,4-bis(1,1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling 2014, 30, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, J.; Karthikeyan, S.; Liu, H.; Cai, J. Natural anti-phytopathogenic fungi compound phenol, 2, 4-bis (1, 1-dimethylethyl) from Pseudomonas fluorescens TL-1. Indian J. Biochem. Biophys. 2019, 56, 162–168. [Google Scholar]
- Bouaziz, F.; Koubaa, M.; Chaabene, M.; Barba, F.J.; Ghorbel, R.E.; Chaabouni, S.E. High Throughput Screening for Bioactive Volatile Compounds and Polyphenols from Almond (Prunus amygdalus) Gum: Assessment of Their Antioxidant and Antibacterial Activities. J. Food Process. Preserv. 2016, 41, e12996. [Google Scholar] [CrossRef]
- Barretto, D.; Vootla, S. GC-MS Analysis of Bioactive Compounds and Antimicrobial Activity of Cryptococcus rajasthanensis Ky627764 Isolated from Bombyx Mori Gut Microflora. Int. J. Adv. Res. 2018, 6, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Girija, S.; Duraipandiyan, V.; Kuppusamy, P.S.; Gajendran, H.; Rajagopal, R. Chromatographic Characterization and GC-MS Evaluation of the Bioactive Constituents with Antimicrobial Potential from the Pigmented Ink of Loligo duvauceli. Int. Sch. Res. Not. 2014, 2014, 820745. [Google Scholar] [CrossRef]
- Gogoi, D.; Bora, G.; Borgohain, R.; Handique, J.G. Antioxidant Capacity and GC-MS Analysis of Hexane, Ethylacetate and Methanol Extracts of Ficus bhotanica–A Potential Folklore Medicinal Plant. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 201–212. [Google Scholar]
- Vanitha, V.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.; Vidhya, E.; Praseetha, P. Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crop. Prod. 2020, 154, 112748. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Manikandan, S.; Lakshmanan, G.M.A.; Ansarali, S. Identification of Bioactive Compounds from Selected Plectranthus Species by Gas Chromatography-Mass Spectroscopy and Fourier Transform Infra-Red Spectroscopy. J. Biol. Act. Prod. Nat. 2017, 7, 438–451. [Google Scholar] [CrossRef]
- Rahnama, K.; Jahanshahi, M.; Nasrollanejad, S.; Fatemi, M.H.; Tabarestani, M.S. Identification of Volatile Organic Compounds from Trichoderma virens (6011) by GC-MS and Separation of a Bioactive Compound via Nanotechnology. Int. J. Eng. 2016, 29, 1347–1353. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Hoppe, H.; Mabinya, L.V.; Nwodo, U.U.; Okoh, A.I. Chemical constituents, antioxidant and cytotoxicity properties of Leonotisleonurus used in the folklore management of neurological disorders in the Eastern Cape, South Africa. 3 Biotech 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Karabay-Yavasoglu, N.U.; Sukatar, A.; Ozdemir, G.; Horzum, Z. Antimicrobial activity of volatile components and various extracts of the red AlgaJania rubens. Phytother. Res. 2007, 21, 153–156. [Google Scholar] [CrossRef]
- Palanna, K.; Narendrappa, T.; Basavaraj, S.; Shreenivasa, K. Efficacy of Fungal and Bacterial Bio-control Agents on Ganoderma Spp. Causing Foot Rot of Arecanut. Int. J. Agric. Innov. Res. 2017, 6, 299–304. [Google Scholar]
- Nguyen, N.H.; Nguyen, T.T.; Ma, P.C.; Ta, Q.T.H.; Duong, T.-H.; Vo, V.G. Potential Antimicrobial and Anticancer Activities of an Ethanol Extract from Bouea macrophylla. Molecules 2020, 25, 1996. [Google Scholar] [CrossRef]
- Perveen, I.; Raza, M.A.; Iqbal, T.; Naz, I.; Sehar, S.; Ahmed, S. Isolation of anticancer and antimicrobial metabolites from Epicoccum nigrum; endophyte of Ferula sumbul. Microb. Pathog. 2017, 110, 214–224. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zayed, M.Z.; Ali, H.M.; El-Kareem, M.S.M.A. Chemical composition, antioxidant and antibacterial activities of extracts from Schinus molle wood branch growing in Egypt. J. Wood Sci. 2016, 62, 548–561. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-T.; Chen, J.-W.; Rathod, J.; Jiang, Y.-Z.; Tsai, P.-J.; Hung, Y.-P.; Ko, W.-C.; Paredes-Sabja, D.; Huang, I.-H. Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front. Microbiol. 2018, 8, 2635. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Rodriguez, T.; Parejo-Palacio, V.; Gonzalez-Delgado, A.D. Technoeconomic sensibility analysis of industrial agar production from red Algae. Chem. Eng. Trans. 2018, 70, 2029–2034. [Google Scholar]
- Gunukula, S.; Klein, S.J.; Pendse, H.P.; DeSisto, W.J.; Wheeler, M.C. Techno-economic analysis of thermal deoxygenation based biorefineries for the coproduction of fuels and chemicals. Appl. Energy 2018, 214, 16–23. [Google Scholar] [CrossRef]
- Han, W.; Hu, Y.; Li, S.; Huang, J.; Nie, Q.; Zhao, H.; Tang, J. Simultaneous dark fermentative hydrogen and ethanol production from waste bread in a mixed packed tank reactor. J. Clean. Prod. 2017, 141, 608–611. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Lee, B.-R.; Sathiyanarayanan, G.; Song, H.-S.; Kim, J.; Jeon, J.-M.; Kim, J.-H.; Park, S.-H.; Yu, J.-H.; Park, K.; et al. Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour. Technol. 2016, 217, 141–149. [Google Scholar] [CrossRef]
- Navarrete-Bolaños, J.L.; Téllez-Martínez, M.G.; Miranda-López, R.; Jiménez-Islas, H. An experimental strategy validated to design cost-effective culture media based on response surface methodology. Prep. Biochem. Biotechnol. 2017, 47, 578–588. [Google Scholar] [CrossRef]
- Ahmad, S.J.; Suhaini, S.; Sidek, H.M.; Basri, D.F.; Zin, N.M. Anti-methicillin resistant Staphylococcus aureus activity and optimal culture condition of Streptomyces sp. SUK 25. Jundishapur J. Microbiol. 2015, 8, e16784. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Romi, W.; Das, R.; Sharma, H.K.; Thakur, D. Antimicrobial potentiality of actinobacteria isolated from two microbiologically unexplored forest ecosystems of Northeast India. BMC Microbiol. 2018, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.Y.; Feng, R.J.; Zhou, D.B.; Pan, Y.Y.; Chen, Y.F.; Wang, F.; Yin, L.Y.; Zhang, Y.D.; Xie, J.H. Optimization of fermentation conditions through response surface methodology for enhanced antibacterial metabolite production by Streptomyces sp. 1-14 from cassava rhizosphere. PLoS ONE 2018, 13, e0206497. [Google Scholar] [CrossRef]
- Kemung, H.M.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities. Molecules 2020, 25, 3545. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, G.; Zhang, J.; Wu, J. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process. Technol. 2017, 156, 454–460. [Google Scholar] [CrossRef]
- Lindenmaier, R.; Williams, S.D.; Sams, R.L.; Johnson, T.J. Quantitative Infrared Absorption Spectra and Vibrational Assignments of Crotonaldehyde and Methyl Vinyl Ketone Using Gas-Phase Mid-Infrared, Far-Infrared, and Liquid Raman Spectra: S-cis vs s-trans Composition Confirmed via Temperature Studies and ab Initio Methods. J. Phys. Chem. A 2017, 121, 1195–1212. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Hung, C.-M. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresour. Technol. 2017, 245, 188–195. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Huang, D.; Zhao, Y.; Feng, J.; Lu, Q.; Pu, Q.; Wang, Y.; Cheng, G.; Wu, M.; et al. CRISPR-cas3 of Salmonella Upregulates Bacterial Biofilm Formation and Virulence to Host Cells by Targeting Quorum-Sensing Systems. Pathogens 2020, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Guihua, Z.; Cao, S.; Zhang, G.; Xiao, Y.; Liu, S.; Shang, Y. Florfenicol-induced Mitochondrial Dysfunction Suppresses Cell Proliferation and Autophagy in Fibroblasts. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Desaulniers, A.B.; Kishore, N.; Adames, K.; Nargang, F.E. Characterization of Single Gene Deletion Mutants Affecting Alternative Oxidase Production in Neurospora crassa: Role of the yvh1 Gene. Microorganisms 2020, 8, 1186. [Google Scholar] [CrossRef]
- Alam, A.; Imam, N.; Ahmed, M.M.; Tazyeen, S.; Tamkeen, N.; Farooqui, A.; Malik, Z.; Ishrat, R. Identification and Classification of Differentially Expressed Genes and Network Meta-Analysis Reveals Potential Molecular Signatures Associated with Tuberculosis. Front. Genet. 2019, 10, 2029–2034. [Google Scholar] [CrossRef]
- Thakur, D.; Bora, T.; Bordoloi, G.; Mazumdar, S. Influence of nutrition and culturing conditions for optimum growth and antimicrobial metabolite production by Streptomyces sp. 201. J. Med Mycol. 2009, 19, 161–167. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Oxidative enzymes from newly local strain Aspergillus iizukae EAN605 using pumpkin peels as a production substrate: Optimized production, characterization, application and techno-economic analysis. J. Hazard. Mater. 2020, 386, 121954. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; CLSI document M100-S26; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Gerlach, A.D.C.L.; Gadea, A.; da Silveira, R.M.B.; Clerc, P.; Lohézic-le Dévéhat, F. The use of anisaldehyde sulfuric acid as an alternative spray reagent in TLC analysis reveals three classes of compounds in the genus Usnea adans.(Parmeliaceae, li-chenized Ascomycota). Preprints 2018. [Google Scholar] [CrossRef]
- She, W.; Ye, W.; Shi, Y.; Zhou, L.; Zhang, Z.; Chen, F.; Qian, P.-Y. A novel chresdihydrochalcone from Streptomyces chrestomyceticus exhibiting activity against Gram-positive bacteria. J. Antibiot. 2020, 73, 429–434. [Google Scholar] [CrossRef]
- Sharma, P.; Kalita, M.C.; Thakur, D. Broad Spectrum Antimicrobial Activity of Forest-Derived Soil Actinomycete, Nocardia sp. PB-52. Front. Microbiol. 2016, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Esmail, G.A.; Duraipandiyan, V.; Arasu, M.V.; Salem-Bekhit, M.M. Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles 2015, 20, 79–90. [Google Scholar] [CrossRef]
- Gopalakrishnan, Y.; Al-Gheethi, A.; Malek, M.A.; Azlan, M.M.; Al-Sahari, M.; Mohamed, R.R.; Alkhadher, S.; Noman, E. Removal of Basic Brown 16 from Aqueous Solution Using Durian Shell Adsorbent, Optimisation and Techno-Economic Analysis. Sustainability 2020, 12, 8928. [Google Scholar] [CrossRef]
- Jones, S.B.; Zhu, Y.; Anderson, D.B.; Hallen, R.T.; Elliott, D.C.; Schmidt, A.J.; Drennan, C. Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading. (No. PNNL-23227); Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2014. [Google Scholar]







| Source | DF | Sum of Squares | Mean Square | F Value | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | 14 | 6.98 | 636.49 | 23.16 | 6.98 | 636.49 | 23.16 | 3.80 | 2.70 | 4.83 | 0.0026 Significant | 0.0180 Significant | 0.0005 Significant |
| Residual error | 22 | 1.84 | 235.51 | 4.80 | 1.84 | 235.51 | 4.80 | ||||||
| Lack-of-fit | 16 | 2.40 | 211.33 | 5.82 | 2.40 | 211.33 | 5.82 | 7.20 | 0.7044 | 2.79 | 0.0112 Significant | 0.7327 Not significant | 0.1053 Not significant |
| Pure error | 6 | 0.3333 | 300.00 | 2.08 | 0.3333 | 300.00 | 2.08 | ||||||
| Total | 36 | ||||||||||||
| Peak no | R. Time | Name of the Compound | Molecular Formula | Molecular Weight (g/mol) | Area % | Quality (%) | Activity | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.799 | n-dodecane | C12H26 | 170 | 0.66 | 76 | Antioxidants, antimicrobial | [14,15] |
| 2 | 9.908 | Eicosane | C20H42 | 283 | 1.38 | 74 | Antibacterial, antifungal | [16] |
| 3 | 10.200 | Phenol, 2,5-bis (1,1-dimethyl ethyl) | C14H22O | 206 | 2.24 | 87 | Antimicrobial | [17,18] |
| 4 | 21.893 | Cetene | C16H32 | 29 | 0.82 | 75 | Antioxidants | [19] |
| 5 | 23.482 | Diethylphthalate | C12H14O4 | 222 | 3.92 | 88 | Antimicrobial | [16] |
| 6 | 25.037 | 2-methyloctacosane | C29H60 | 409 | 0.81 | 94 | Antifungal | [20] |
| 7 | 25.330 | 1-octadecane | C18H36 | 252 | 0.86 | 75 | Antifungal | [21] |
| 8 | 28.775 | Phthalic acid, isobutyl nonyl ester | C21H32O4 | 348 | 1.11 | 79 | Antimicrobial, antioxidants | [22] |
| 9 | 29.896 | Heneicosane | C21H44 | 297 | 1.86 | 74 | Antibacterial | [23] |
| 10 | 31.735 | n-hexa-decanoic acid | C16H32O2 | 256 | 1.02 | 73 | Cosmetics, antioxidants | [24,25] |
| Peak no | R. Time | Name of the Compound | Molecular Formula | Molecular Weight (g/mol) | Area % | Quality (%) | Activity | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 17.671 | Di-butyl phthalate | C16H22O4 | 278 | 2.64 | 86 | Antifungal | [26] |
| 2 | 12.301 | 1-nonadecene | C19H38 | 267 | 0.85 | 79 | Antioxidants, antimicrobial | [27] |
| 3 | 12.734 | Thieno[3,2-e] benzofuran | C10H6OS | 174 | 1.20 | 92 | Antibacterial | [28] |
| 4 | 13.279 | (1-decene) | C10H20 | 140 | 0.46 | 85 | Antifungal | [29] |
| 5 | 13.509 | Diisooctyl phthalate | C24H38O4 | 391 | 1.11 | 98 | Anticancer, antibacterial | [30] |
| 6 | 13.590 | Bis (2-ethyl hexyl) phthalate | C24H38O4 | 391.56 | 79.06 | 94 | Antimicrobial | [31] |
| 7 | 14.084 | Dodecane | C12H26 | 170 | 19.41 | 69 | neurotropic action | [14,15] |
| 8 | 17.762 | Eicosane | C20H42 | 283 | 5.31 | 71 | Antimicrobial, Antifungal | [16] |
| 9 | 18.532 | Heneicosane | C21H44 | 296 | 3.70 | 72 | Antifungal | [23] |
| 10 | 19.648 | 1,2-benzenedi carboxylic acid | C8H6O4 | 166.14 | 3.1 | 93 | Antimicrobial and anti-inflammatory Activities | [32] |
| 11 | 25.431 | Lauric acid, dodecanoic acid | C12H24O2 | 200.32 | 2.1 | 74 | Antimicrobial and anti-inflammatory Activities | [33] |
| Item Code * | Quantity | Item | Percentage of FCE | Cost |
|---|---|---|---|---|
| P-1/BR-101 | 1 | Cell culture bio-reactor | 33.33% | 10,000.00 |
| P-1/DS-101 | 2 | Centrifuge | 30,000.00 | |
| P-1/MSX-101 | 1 | Purification system | 17,000.00 | |
| P-1/FDR-101 | 1 | Freeze drying | 5000.00 | |
| P-1/RBS-101 | 1 | Inoculum preparation | 5000.00 | |
| P-2/V-101 | 2 | Storage tank | 25,000.00 | |
| P-1/MF-101 | 1 | Microfiltration | 14,000.00 | |
| P-1/AB-101 | 1 | Aerobic bio-oxidation | 30,000.00 | |
| P-2/DE-101 | 1 | Dead end filtration | 20,000.00 | |
| P-1/DB-101 | 1 | Biomass storage tank | 13,000.00 | |
| Total equipment purchase cost | 169,000.00 | |||
| Equipment installation | 9.86% | 50,000.00 | ||
| Process piping | 9.86% | 50,000.00 | ||
| Instrumentation and controls | 9.47% | 48,000.00 | ||
| Electrical systems | 9.86% | 50,000.00 | ||
| Buildings | 7.89% | 40,000.00 | ||
| Yard improvements | 3.94% | 20,000.00 | ||
| Construction | 15.78% | 80,000.00 | ||
| TOTAL | 507,000.00 | |||
| Component | Price (U.S.D) | Unit | Quantity | Total Cost (U.S.D) | |
|---|---|---|---|---|---|
| Raw material | K2HPO4 | 80 | kg | 1000 kg | 80,000.00 |
| (chemicals) | KNO3 | 100 | kg | 500 kg | 50,000.00 |
| MgSO4.2H2O | 300 | kg | 200 kg | 60,000.00 | |
| CaCl2.H2O | 100 | kg | 100 kg | 10,000.00 | |
| NaCl | 100 | kg | 100 kg | 10,000.00 | |
| FeCl3 | 350 | kg | 10 kg | 3500 | |
| Glucose | 415 | kg | 1500 kg | 62,200.00 | |
| Asparagine | 1500 | kg | 50 kg | 75,000.00 | |
| HCl | 100/L | 1 L | 100 L | 10,000.00 | |
| NaOH | 160/L | 1 L | 50 L | 8000 | |
| Reagents required for the purification | 20,000.00 | ||||
| Reagents required for the application test | 50,000.00 | ||||
| Utilities | Electricity | 0.04 | kWh | 1,000,000 | 40,000.00 |
| Water | 0.01 | U.S.D/m3 | 6,000,000 | 60,000.00 | |
| Other costs | Labor | 12000 | U.S.D/ | 10 | 120,000.00 |
| employee | |||||
| Maintenance | 2 | % of FCE | 5070 | ||
| Insurance | 1 | % of FCE | 10,140.00 | ||
| Total | 673,910.00 | ||||
| Medium Components | Codes | High Level (+) | Medium Level | Low Level (–) |
|---|---|---|---|---|
| Incubation Time (day) | 21 | 12 | 0 | |
| pH | 9 | 6 | 4 | |
| Temperature °C | 26 | 30 | 34 | |
| Shaker rotation speed (rpm) | 180 | 160 | 120 | |
| Glucose (g/L) | 3 | 2 | 1 | |
| Mannitol (g/L) | 3 | 2 | 1 | |
| Asparagine (g/L) | 2 | 1.0 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shaibani, M.M.; Radin Mohamed, R.M.S.; Zin, N.M.; Al-Gheethi, A.; Al-Sahari, M.; El Enshasy, H.A. Enhanced Pharmaceutically Active Compounds Productivity from Streptomyces SUK 25: Optimization, Characterization, Mechanism and Techno-Economic Analysis. Molecules 2021, 26, 2510. https://doi.org/10.3390/molecules26092510
Al-Shaibani MM, Radin Mohamed RMS, Zin NM, Al-Gheethi A, Al-Sahari M, El Enshasy HA. Enhanced Pharmaceutically Active Compounds Productivity from Streptomyces SUK 25: Optimization, Characterization, Mechanism and Techno-Economic Analysis. Molecules. 2021; 26(9):2510. https://doi.org/10.3390/molecules26092510
Chicago/Turabian StyleAl-Shaibani, Muhanna Mohammed, Radin Maya Saphira Radin Mohamed, Noraziah Mohamad Zin, Adel Al-Gheethi, Mohammed Al-Sahari, and Hesham Ali El Enshasy. 2021. "Enhanced Pharmaceutically Active Compounds Productivity from Streptomyces SUK 25: Optimization, Characterization, Mechanism and Techno-Economic Analysis" Molecules 26, no. 9: 2510. https://doi.org/10.3390/molecules26092510