Antidiabetic and Renoprotective Effects of Coffea arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats
Abstract
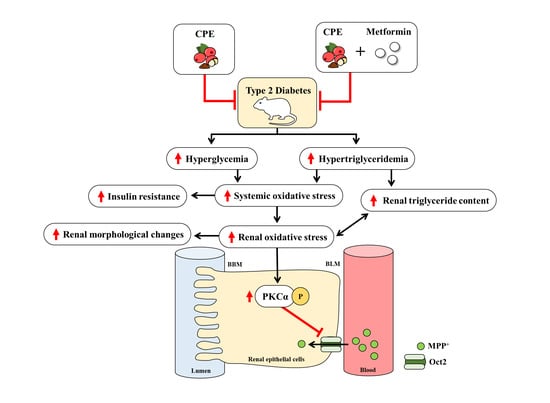
:1. Introduction
2. Results
2.1. Effect of CPE on Selected Overall Nutritional, Growth, and Biochemical Parameters of Carbohydrate and Lipid Metabolism as Well as General Kidney Function in Rats
2.2. Effect of CPE on Pathological Renal Damage
2.3. Effect of CPE on Renal Lipid Content and Lipid Peroxidation
2.4. Effect of CPE on Impairment of Renal Organic Cation Transport Function
2.5. Effect of CPE on Renal Antioxidative Gene Expression
2.6. Effect of CPE on Stress-Sensitive Signaling
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Coffea arabica Pulp Aqueous Extract Preparation and Total Phenolic Content Measurement
4.3. Animals and Induction of Experimental Type 2 Diabetic Rats
4.4. Biochemical Analysis
4.5. Histological Analysis
4.6. Renal Slice Preparation and Transport Study
4.7. Renal Triglyceride Accumulation and Lipid Peroxidation
4.8. Antioxidant Gene Expression Using Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.9. Subcellular Fractions and Western Blot Analysis
4.10. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ABCA1 | Adenosine triphosphate binding cassette (ABC) transporter A1 |
| BW | Body weight |
| CGA | Chlorogenic acid |
| CD36 | Cluster of differentiation 36 |
| CP | Coffea arabica pulp |
| CPE | Coffea arabica pulp aqueous extract |
| cDNA | Complementary deoxyribonucleic acid |
| CuZn-SOD | Copper-zinc superoxide dismutase |
| EC | Epicatechin |
| ES | Estrone sulfate |
| GPx | Glutathione peroxidase |
| HOMA index | Homeostasis assessment of insulin resistance index |
| LXR | Liver X receptor |
| MDA | Malondialdehyde |
| MPP+ | 1-methyl-4-phenylpyridinium |
| ND | Normal diet |
| Oat1 | Organic anion transporter 1 |
| Oat3 | Organic anion transporter 3 |
| Oct2 | Organic cation transporter 2 |
| PAH | Para-aminohippurate |
| p-PKC | Phosphorylated PKC |
| qPCR | Quantitative real-time polymerase chain reaction |
| RNA | Ribonucleic acid |
| Na+-K+-ATPase | Sodium potassium ATPase |
| SREBP-1 | Sterol regulatory element-binding protein 1 |
| STZ | Streptozotocin |
| TBS-T | Tris-buffered saline |
| T2D | Type 2 diabetes |
| VFW | Visceral fat weight |
References
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Juster-Switlyk, K.; Smith, A.G. Updates in diabetic peripheral neuropathy. F1000Research 2016, 5, 738. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Lai, K.N. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol. Dial. Transplant. 2012, 27, 3049–3056. [Google Scholar] [CrossRef] [Green Version]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free. Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Darshi, M.; Van Espen, B.; Sharma, K. Metabolomics in diabetic kidney disease: Unraveling the biochemistry of a silent killer. Am. J. Nephrol. 2016, 44, 92–103. [Google Scholar] [CrossRef]
- Zeni, L.; Norden, A.G.W.; Cancarini, G.; Unwin, R.J. A more tubulocentric view of diabetic kidney disease. J. Nephrol. 2017, 30, 701–717. [Google Scholar] [CrossRef]
- Nigam, S.K. The SLC22 Transporter Family: A paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Renal organic anion transporters (SLC22 Family): Expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013, 15, 53–69. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Inui, K.-I. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 2013, 15, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manautou, J.; Nowicki, M.; Aleksunes, L.; Sawant, S.; Dnyanmote, A.; Mehendale, H. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab. Lett. 2008, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Tikellis, C.; Burns, W.C.; Thallas, V.; Forbes, J.M.; Cao, Z.; Osicka, T.M.; Russo, L.M.; Jerums, G.; Ghabrial, H.; et al. Reduced tubular cation transport in diabetes: Prevented by ACE inhibition. Kidney Int. 2003, 63, 2152–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, A.; Mateus, N.; De Freitas, V.; Calhau, C. Modulation of MPP+ uptake by procyanidins in Caco-2 cells: Involvement of oxidation/reduction reactions. FEBS Lett. 2005, 580, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Ontawong, A.; Saowakon, N.; Vivithanaporn, P.; Pongchaidecha, A.; Lailerd, N.; Amornlerdpison, D.; Lungkaphin, A.; Srimaroeng, C. Antioxidant and renoprotective effects of Spirogyra neglecta (Hassall) Kützing extract in experimental type 2 diabetic rats. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.; Manjunatha, M.R.; Sulochannama, G.; Naidu, M. Extraction, characterization and bioactivity of coffee anthocyanins. Eur. J. Biol. Sci. 2012, 4, 13–19. [Google Scholar] [CrossRef]
- Ramirez-Martinez, J.R. Phenolic compounds in coffee pulp: Quantitative determination by HPLC. J. Sci. Food Agric. 1988, 43, 135–144. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [Green Version]
- Galvano, F.; La Fauci, L.; Vitaglione, P.; Fogliano, V.; Vanella, L.; Felgines, C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann. Ist. Super. Sanità 2007, 43, 382–393. [Google Scholar]
- Ontawong, A.; Boonphang, O.; Pasachan, T.; Duangjai, A.; Pongchaidecha, A.; Phatsara, M.; Jinakote, M.; Amornlerdpison, D.; Srimaroeng, C. Hepatoprotective effect of coffee pulp aqueous extract combined with simvastatin against hepatic steatosis in high-fat diet-induced obese rats. J. Funct. Foods 2019, 54, 568–577. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Muanprasat, C.; Pasachan, T.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 2019, 52, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Manna, P.; Gachhui, R.; Sil, P.C. d-Saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-κB and PKC signaling. Toxicol. Appl. Pharmacol. 2013, 267, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, E.Y.; Choi, R.; Nawaboot, J.; Lee, M.Y.; Kim, H.S.; Chung, C.H. Protective effects of curcumin on renal oxidative stress and lipid metabolism in a rat model of type 2 diabetic nephropathy. Yonsei Med. J. 2016, 57, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.; Kim, D.; Kim, J.S. Body Fat Distribution and the risk of incident metabolic syndrome: A longitudinal cohort study. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose Response 2017, 15, 1559325817691158. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A Catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; De Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; De Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [Green Version]
- Xu-Feng, H.; Yu, Y.-H.; Wang, S.-T.; Ren, J.; Camer, D.; Hua, Y.-Z.; Zhang, Q.; Huang, J.; Xue, D.-L.; Zhang, X.-F.; et al. Chlorogenic acid protectsd-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Lou, J.; Gu, X.; Xing, Y.; Cui, J.; Lv, W.; Zhang, Y. Chlorogenic acid slows down proteinuria and renal fibrosis in 5/6-nephrectomized rats by anti-oxidation and inhibiting accumulation of extracellular matrix. Int. J. Clin. Exp. Med. 2016, 9, 15719–15727. [Google Scholar]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Halaihel, N.; Zhang, W.; Rogers, T.; Levi, M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in Diabetes Mellitus. J. Biol. Chem. 2002, 277, 18919–18927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsun, J.G.S.; Yung, S.; Chau, M.K.M.; Shiu, S.W.M.; Chan, T.M.; Tan, K.C.B. Cellular cholesterol transport proteins in diabetic nephropathy. PLoS ONE 2014, 9, e105787. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, P.G.; Pannakal, S.T.; Priyadarsini, K.; Unnikrishnan, M. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 2012, 64, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-F.; Cho, S.; Wang, J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann. Clin. Transl. Neurol. 2014, 1, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, M.; Ai, Q.; Lin, L.; Wu, K.; Deng, X.; Jing, Y.; Jia, M.; Wan, J.; Zhang, L. Involvement of catalase in the protective benefits of metformin in mice with oxidative liver injury. Chem. Biol. Interact. 2014, 216, 34–42. [Google Scholar] [CrossRef]
- Wright, S.H.; Dantzler, W.H. Molecular and cellular physiology of renal organic cation and anion transport. Physiol. Rev. 2004, 84, 987–1049. [Google Scholar] [CrossRef] [PubMed]
- Mehrens, T.; Lelleck, S.; Cetinkaya, I.; Knollmann, M.; Hohage, H.; Gorboulev, V.; Bokník, P.; Koepsell, H.; Schlatter, E. The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J. Am. Soc. Nephrol. 2000, 11, 1216–1224. [Google Scholar]
- Urakami, Y.; Okuda, M.; Masuda, S.; Akazawa, M.; Saito, H.; Inui, K. Distinct characteristics of organic cation transporters, OCT1 and OCT2, in the basolateral membrane of renal tubules. Pharm. Res. 2001, 18, 1528–1534. [Google Scholar] [CrossRef]
- Pietig, G.; Mehrens, T.; Hirsch, J.R.; Çetinkaya, I.; Piechota, H.; Schlatter, E. Properties and regulation of organic cation transport in freshly isolated human proximal tubules. J. Biol. Chem. 2001, 276, 33741–33746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brast, S.; Grabner, A.; Sucic, S.; Sitte, H.H.; Hermann, E.; Pavenstädt, H.; Schlatter, E.; Ciarimboli, G. The cysteines of the extracellular loop are crucial for trafficking of human organic cation transporter 2 to the plasma membrane and are involved in oligomerization. FASEB J. 2012, 26, 976–986. [Google Scholar] [CrossRef] [Green Version]
- DelVecchio, C.J.; Capone, J.P. Protein kinase C α modulates liver X receptor α transactivation. J. Endocrinol. 2008, 197, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Wongwan, T.; Kittayaruksakul, S.; Asavapanumas, N.; Chatsudthipong, V.; Soodvilai, S. Activation of liver X receptor inhibits OCT2-mediated organic cation transport in renal proximal tubular cells. Pflüg. Arch. 2017, 469, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.-Y.; Li, Z.-Y.; Zheng, Y.; Chen, Y.; Zhou, Z.-H.; Jin, J. The attenuation of chlorogenic acid on oxidative stress for renal injury in streptozotocin-induced diabetic nephropathy rats. Arch. Pharmacal Res. 2016, 39, 989–997. [Google Scholar] [CrossRef]
- Kulkarni, C.R.; Joglekar, M.M.; Patil, S.B.; Arvindekar, A.U. Antihyperglycemic and antihyperlipidemic effect of Santalum albumin streptozotocin induced diabetic rats. Pharm. Biol. 2012, 50, 360–365. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Boysen, G.; Li, F.; Li, Y.; Swenberg, J.A. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007, 71, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Rose, H.G.; Oklander, M. Improved procedure for the extraction of lipids from human erythrocytes. J. Lipid Res. 1965, 6, 428–431. [Google Scholar] [CrossRef]





| Parameters | ND | ND + CPE | DM | DM + CPE | DM + Met | DM + CPE + Met |
|---|---|---|---|---|---|---|
| Food Intake (g/day) | 19.94 ± 0.67 | 17.99 ± 0.41 | 22.68 ± 1.03 * | 21.21 ± 0.90 | 22.45 ± 0.81 * | 20.66 ± 0.89 |
| Calories Intake (kcal/day) | 80.11 ± 2.68 | 72.17 ± 1.65 | 121.31 ± 5.50 * | 111.90 ± 4.80 * | 119.34 ± 4.40 * | 119.20 ± 6.94 * |
| Water Intake (mL/day) | 37.96 ± 2.14 | 44.04 ± 3.80 | 44.62 ± 4.08 | 46.92 ± 1.97 | 46.41 ± 1.83 | 40.94 ± 2.07 |
| BW (g) | 476.88 ± 9.91 | 448.75 ± 9.85 | 510.00 ± 27.12 | 468.33 ± 26.07 | 475.83 ± 32.21 | 464.38 ± 25.76 |
| BW Gain (g) | 136.25 ± 10.97 | 66.25 ± 17.13 * | 130.00 ± 18.55 | 109.17 ± 13.44 | 125.83 ± 30.53 | 93.75 ±25.70 |
| VFW/BW (g/100 g BW) | 7.08 ± 0.35 | 4.74 ± 0.34 * | 9.78 ± 0.88 * | 7.05 ± 0.33 # | 8.07 ± 0.86 | 6.45 ± 0.66 # |
| Relative KW | 5.22 ± 0.14 | 5.44 ± 0.10 | 5.59 ± 0.37 | 6.13 ± 0.27 | 6.14 ± 0.65 | 5.98 ± 0.41 |
| Parameters | ND | ND + CPE | DM | DM + CPE | DM + Met | DM + CPE + Met |
|---|---|---|---|---|---|---|
| Plasma Glucose (mg/dL) | 133.25 ±3.14 | 132.71 ± 2.61 | 428.47 ± 37.27 * | 237.45 ± 19.76 *,# | 218.43 ± 28.06 *,# | 231.10 ± 28.70 *,# |
| Plasma Triglycerides (mg/dL) | 42.43 ± 4.41 | 42.23 ± 5.93 | 80.12 ± 7.93 * | 43.82 ± 6.92 # | 50.69 ± 4.43 # | 40.73 ± 5.08 # |
| Plasma insulin (ng/mL) | 2.33 ± 0.35 | 2.81 ± 0.96 | 2.66 ± 0.50 | 1.89 ± 0.41 | 1.82 ± 0.35 | 1.97 ± 0.29 |
| HOMA Index | 20.13 ± 3.19 | 23.21 ± 7.97 | 72.19 ± 14.42 * | 29.05 ± 6.94 # | 26.54 ± 5.35 # | 26.14 ± 5.46 # |
| Estimated GFR (mL/min) | 1.30 ± 0.60 | 1.12 ± 0.34 | 1.30 ± 0.27 | 1.31 ± 0.18 | 1.37 ± 0.25 | 1.26 ± 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonphang, O.; Ontawong, A.; Pasachan, T.; Phatsara, M.; Duangjai, A.; Amornlerdpison, D.; Jinakote, M.; Srimaroeng, C. Antidiabetic and Renoprotective Effects of Coffea arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats. Molecules 2021, 26, 1907. https://doi.org/10.3390/molecules26071907
Boonphang O, Ontawong A, Pasachan T, Phatsara M, Duangjai A, Amornlerdpison D, Jinakote M, Srimaroeng C. Antidiabetic and Renoprotective Effects of Coffea arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats. Molecules. 2021; 26(7):1907. https://doi.org/10.3390/molecules26071907
Chicago/Turabian StyleBoonphang, Oranit, Atcharaporn Ontawong, Tipthida Pasachan, Manussabhorn Phatsara, Acharaporn Duangjai, Doungporn Amornlerdpison, Metee Jinakote, and Chutima Srimaroeng. 2021. "Antidiabetic and Renoprotective Effects of Coffea arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats" Molecules 26, no. 7: 1907. https://doi.org/10.3390/molecules26071907