Facile Electrochemical Method for the Fabrication of Stable Corrosion-Resistant Superhydrophobic Surfaces on Zr-Based Bulk Metallic Glasses
Abstract
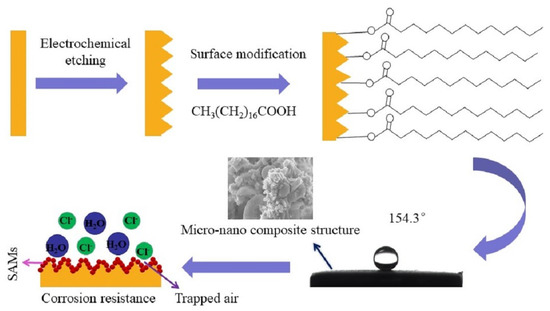
:1. Introduction
2. Experimental
2.1. Preparation of Zr-Based Metallic Glasses
2.2. Fabrication of Superhydrophobic Surfaces
2.3. Sample Characterisation
3. Results and Discussion
3.1. Wettability Analysis and Surface Morphology
3.2. Chemical Composition and Surface Chemical Reaction Process
3.3. Corrosion Resistance
3.4. Chemical Stability
3.5. Self-Cleaning Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Yin, X.; Hu, J.; Yu, S.; Zhao, Y.; Xiong, W. Fabrication of a biomimetic hierarchical superhydrophobic Cu-Ni coating with self-cleaning and anti-corrosion properties. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124223. [Google Scholar] [CrossRef]
- Chi, F.; Liu, D.; Wu, H.; Lei, J. Mechanically robust and self-cleaning antireflection coatings from nanoscale binding of hydrophobic silica nanoparticles. Sol. Energy Mater. Sol. Cells 2019, 200, 109939. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloys Compd. 2008, 765, 721–730. [Google Scholar] [CrossRef]
- Balordi, M.; Cammi, A.; de Magistris, G.S.; Chemelli, C. Role of micrometric roughness on anti-ice properties and durability of hierarchical super-hydrophobic aluminum surfaces. Surf. Coat. Technol. 2019, 374, 549–556. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Zhang, Y. Superhydrophobic Copper Tubes with Possible Flow Enhancement and Drag Reduction. ACS Appl. Mater. Interfaces 2009, 1, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Nguyen, D.C.; Kim, D.; Hwang, W.; Yoon, B. Experimental drag reduction study of super-hydrophobic surface with dual-scale structures. Appl. Surf. Sci. 2013, 286, 206–211. [Google Scholar] [CrossRef]
- Chen, J.; Guo, D.; Huang, C.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. Fabrication of superhydrophobic copper mesh by depositing CuCl for oil/water separation. Mater. Lett. 2018, 233, 328–331. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Z.; Shen, Y.; Mu, P.; Zhu, G.; Li, J. Facile fabrication of superhydrophobic copper hydroxide coated mesh for effective separation of water-in-oil emulsions. Sep. Purif. Technol. 2020, 230, 115856. [Google Scholar] [CrossRef]
- Lahann, J.; Mitragotri, S.; Tran, T.-N.; Kaido, H.; Sundaram, J.; Choi, I.S.; Hoffer, S.; Somorjai, G.A.; Langer, R. A Reversibly Switching Surface. Science 2003, 299, 371–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, W.; Wu, J.; Gu, T.; Wang, P.; Zhang, D. Preparation of super-hydrophobic micro-needle CuO surface as a barrier against marine atmospheric corrosion. Corros. Sci. 2018, 131, 156–163. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Jing, Z.; Yan, L.; Zha, F.; Lei, Z. One-step spray-coating process for the fabrication of colorful superhydrophobic coatings with excellent corrosion resistance. Langmuir 2015, 31, 10702–10707. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Wu, J.; Tan, L.; Zhang, B.; Yang, K. Research on super-hydrophobic surface of biodegradable magnesium alloys used for vascular stents. Mater. Sci. Eng. C 2013, 33, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Xu, W.; Carmalt, C.J.; Parkin, I.P. Water droplets bouncing on superhydrophobic soft porous materials. J. Mater. Chem. A 2014, 2, 12177–12184. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Lei, Y.; Wang, Y.; Zhou, G.; Xu, C.; Rao, Y.; Wang, K. Preparation of intricate nanostructures on 304 stainless steel surface by SiO2-assisted HF etching for high superhydrophobicity. Colloids Surf. A 2020, 586, 124287. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Guo, D. Facile fabrication of superhydrophobic surfaces with low roughness on Ti–6Al–4V substrates via anodization. Appl. Surf. Sci. 2014, 314, 754–759. [Google Scholar] [CrossRef]
- Wu, H.; Xie, L.; Zhang, R.; Tian, Y.; Liu, S.; He, M.; Huang, C.; Tian, W. A novel method to fabricate organic-free superhydrophobic surface on titanium substrates by removal of surface hydroxyl groups. Appl. Surf. Sci. 2019, 479, 1089–1097. [Google Scholar] [CrossRef]
- Ding, C.; Tai, Y.; Wang, D.; Tan, L.; Fu, J. Superhydrophobic composite coating with active corrosion resistance for AZ31B magnesium alloy protection. Chem. Eng. J. 2019, 357, 518–532. [Google Scholar] [CrossRef]
- Gray-Munro, J.; Campbell, J. Mimicking the hierarchical surface topography and superhydrophobicity of the lotus leaf on magnesium alloy AZ31. Mater. Lett. 2017, 189, 271–274. [Google Scholar] [CrossRef]
- Mandal, P.; Ivvala, J.; Arora, H.S.; Grewal, H.S. Sustainable approach for the development of durable superhydrophobic metallic surfaces. Mater. Lett. 2020, 279, 128516. [Google Scholar] [CrossRef]
- He, A.; Liu, W.; Xue, W.; Yang, H.; Cao, Y. Nanosecond laser ablated copper superhydrophobic surface with tunable ultrahigh adhesion and its renewability with low temperature annealing. Appl. Surf. Sci. 2018, 434, 120–125. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, C.; Liu, R.; Zhang, Q.; Gong, J.; Tao, D.; Ji, Z. Fabrication of superhydrophobic surface with enhanced corrosion resistance on H62 brass substrate. Colloids Surf. A 2020, 589, 124475. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Trexler, M.M.; Thadhani, N.N. Mechanical properties of bulk metallic glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, J.; Yu, M.; Zhang, M.; Liu, F.; Zhang, Y.; Liu, L. Effects of heat treatment on the thermal, mechanical and corrosion properties of deformed Zr-based bulk metallic glasses. Mater. Chem. Phys. 2020, 256, 123705. [Google Scholar] [CrossRef]
- Li, N.; Xia, T.; Heng, L.; Liu, L. Superhydrophobic Zr-based metallic glass surface with high adhesive force. Appl. Phys. Lett. 2013, 102, 251603. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.Y.; Wang, D.P.; Zhao, D.Q.; Ding, D.W.; Liu, K.; Wang, W.H. Superhydrophobic metallic glass surface with superior mechanical stability and corrosion resistance. Appl. Phys. Lett. 2014, 104, 173701. [Google Scholar] [CrossRef]
- Qiao, J.H.; Jin, X.; Qin, J.H.; Liu, H.T.; Luo, Y.; Zhang, D.K. A super-hard superhydrophobic Fe-based amorphous alloy coating. Surf. Coat. Technol. 2018, 334, 286–291. [Google Scholar] [CrossRef]
- Jiang, Q.K.; Wang, X.D.; Nie, X.P.; Zhang, G.Q.; Ma, H.; Fecht, H.J.; Bendnarcik, J.; Franz, H.; Liu, Y.G.; Cao, Q.P.; et al. Zr-(Cu, Ag)–Al bulk metallic glasses. Acta Mater. 2008, 56, 1785–1796. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.S.; Zhang, W.; Kai, W.; Liaw, P.K.; Huang, H.H. Evaluation of Ni-free Zr–Cu–Fe–Al bulk metallic glass for biomedical implant applications. J. Alloys Compd. 2014, 586, S539–S543. [Google Scholar] [CrossRef]
- Li, T.; Wong, P.; Chang, S.; Tsai, P.; Jang, J.; Huang, J. Biocompatibility study on Ni-free Ti-based and Zr-based bulk metallic glasses. Mater. Sci. Eng. C 2017, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wang, L.L.; Sun, Y.W.; Gao, Y.Z.; Guo, D.M. Preparation of durable underwater superoleophobic Ti6Al4V surfaces by electrochemical etching. Surf. Eng. 2016, 32, 85–94. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Gebert, A.; Schultz, L. Pitting corrosion of Cu–Zr metallic glasses in hydrochloric acid solutions. J. Alloy. Compd. 2008, 462, 60–67. [Google Scholar] [CrossRef]
- Xie, X.; Ni, C.; Lin, Z.; Wu, D.; Sun, X.; Zhang, Y.; Wang, B.; Du, W. Phase and morphology evolution of high dielectric CoO/Co3O4 particles with Co3O4 nanoneedles on surface for excellent microwave absorption application. Chem. Eng. J. 2020, 396, 125205. [Google Scholar] [CrossRef]
- Mokhtari, S.; Karimzadeh, F.; Abbasi, M.; Raeissi, K. Development of super-hydrophobic surface on Al 6061 by anodizing and the evaluation of its corrosion behavior. Surf. Coatings Technol. 2017, 324, 99–105. [Google Scholar] [CrossRef]
- Jadhav, S.A. Self-assembled monolayers (SAMs) of carboxylic acids: An overview. Cent. Eur. J. Chem. 2011, 9, 369–378. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J.; Zhang, X.; Yan, H.; Yang, W.; Liu, Q.; Zhang, M.; Liu, L.; Takahashi, K. Fabrication of superhydrophobic magnesium alloy through the oxidation of hydrogen peroxide. Colloids Surf. A. 2013, 436, 906–911. [Google Scholar] [CrossRef]
- Barnartt, S. Two-point and three-point methods for the investigation of electrode reaction mechanisms. Electrochim. Acta 1970, 15, 1313–1324. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves. Corros. Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Jeong, C.; Lee, J.; Sheppard, K.; Choi, C.-H. Air-Impregnated Nanoporous Anodic Aluminum Oxide Layers for Enhancing the Corrosion Resistance of Aluminum. Langmuir 2015, 31, 11040–11050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, J.; Luo, D.; Wang, H.; Gong, X.; Han, Z.; Ren, L. One-step fabrication of biomimetic superhydrophobic surface by electrodeposition on magnesium alloy and its corrosion inhibition. J. Colloid Interface Sci. 2017, 491, 313–320. [Google Scholar] [CrossRef] [PubMed]






| Etching Time (min) | Roughness (μm) | CAs after Electrochemical Etching | CAs after Surface Modification | Sliding Angle |
|---|---|---|---|---|
| 0 | 0.452 | 75.3° ± 1.2° | 106.7° ± 1.5° | > 10° |
| 2 | 0.692 | 108.6° ± 4.2° | 138.6° ± 3.2° | > 10° |
| 7 | 2.303 | 114.3° ± 2.9° | 153.4°± 2.5° | < 5° |
| 12 | 3.550 | 119.4° ± 4.3° | 153.2° ± 3.0° | < 5° |
| 15 | 4.566 | 116.3° ± 3.9° | 154.3° ± 2.2° | < 5° |
| 17 | 4.916 | 123.1° ± 5.8° | 152.4° ± 2.4° | < 5° |
| 22 | 5.322 | 116.3° ± 3.3° | 151.2° ± 0.9° | < 5° |
| Electrochemical Etching Time (min) | Ecorr (V) | Icorr (A cm−2) | η (%) |
|---|---|---|---|
| 0 | −0.472 | 1.080 × 10−5 | - |
| 7 | −0.370 | 1.376 × 10−6 | 87.3 |
| 15 | −0.280 | 3.920 × 10−8 | 99.6 |
| 22 | −0.415 | 2.160 × 10−6 | 80.0 |
| Etching Time (min) | Rs (Ω⋅cm2) | QCPE (Ω−1s−n cm−2) | n | R1 (Ω⋅cm2) |
|---|---|---|---|---|
| 0 | 390.4 | 3.48′ 10−6 | 0.567 | 16,084 |
| 15 | 593.2 | 6.83 × 10−7 | 0.659 | 85,518 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Zhang, M.; Sun, J.; Liu, F.; Wang, Y.; Ding, G.; Xie, X.; Liu, L.; Zhao, X.; Li, H. Facile Electrochemical Method for the Fabrication of Stable Corrosion-Resistant Superhydrophobic Surfaces on Zr-Based Bulk Metallic Glasses. Molecules 2021, 26, 1558. https://doi.org/10.3390/molecules26061558
Yu M, Zhang M, Sun J, Liu F, Wang Y, Ding G, Xie X, Liu L, Zhao X, Li H. Facile Electrochemical Method for the Fabrication of Stable Corrosion-Resistant Superhydrophobic Surfaces on Zr-Based Bulk Metallic Glasses. Molecules. 2021; 26(6):1558. https://doi.org/10.3390/molecules26061558
Chicago/Turabian StyleYu, Mengmeng, Ming Zhang, Jing Sun, Feng Liu, Yujia Wang, Guanzhong Ding, Xiubo Xie, Li Liu, Xiangjin Zhao, and Haihong Li. 2021. "Facile Electrochemical Method for the Fabrication of Stable Corrosion-Resistant Superhydrophobic Surfaces on Zr-Based Bulk Metallic Glasses" Molecules 26, no. 6: 1558. https://doi.org/10.3390/molecules26061558