Modified Nanofibrous Filters with Durable Antibacterial Properties
Abstract
:1. Introduction
2. Results
2.1. Influence of Micro- and Nanoparticles of CuO on the Properties of the PU Solution
2.1.1. Viscosity
2.1.2. Conductivity and Surface Tension
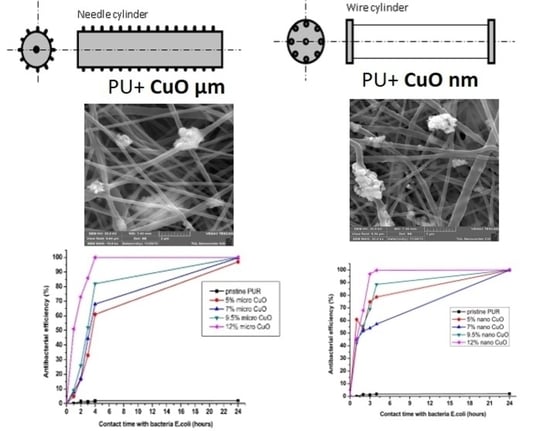
2.2. Structure of Produced Composite Nanofibers
2.3. Antibacterial Properties of Composite Nanofibrous Layers
2.4. Antibacterial Air Filtration Efficiency
2.5. Stability of Antibacterial Properties of Modified Nanofibers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Properties of the Solution
4.3. Electrospinning Process—Used Spinning Electrodes
4.3.1. The Roller Spinning Method
- voltage = 67 kV
- roller speed = 2.5 rpm
- speed of collecting material = 0.05 m/min
- distance between the rotating cylinder and collector electrode = 16 cm
- temperature (T °C) = 20 °C
- humidity in the spinning chamber = 22%.
4.3.2. Wire Spinning Electrode
- voltage = 60 kV
- traversing speed of wire = 0.2 mm/s; speed of collecting material = 0.05 m/min
- distance between the wire and collector electrode = 17.5 cm
- temperature (T °C) = 20 °C
- humidity in the spinning chamber = 22%.
4.4. Structure of Produced Materials
4.5. Antibacterial Properties of Composite Nanofibrous Layers
4.6. Stability of Particles Fixation into the Nanofibrous Structure
4.7. Measurement of Bacterial Filtration Efficiency
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fang, J.; Wang, X.; Lin, T. Functional Applications of Electrospun Nanofibers. Nanofibers Prod. Prop. Funct. Appl. 2011, 14, 287–302. [Google Scholar]
- Graham, K.; Ouyang, M.; Raether, T.; Grafe, T.; Mcdonald, B.; Knauf, P. Polymeric Nanofibers in Air Filtration Applications. In Proceedings of the 15th Annual Technical Conference & Expo of the American Filtration & Separations Society, Galveston, TX, USA, 9 April 2002; pp. 9–12. [Google Scholar]
- Pant, H.R.; Kim, H.J.; Joshi, M.K.; Pant, B.; Park, C.H.; Kim, J.I.; Hui, K.S.; Kim, C.S. One-step fabrication of multifunctional composite polyurethane spider-web-like nanofibrous membrane for water purification. J. Hazard. Mater. 2014, 264, 25–33. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Liu, Y.B.; Yao, J.B. A Review on Existing Tecgnology of Electrospinning at Large Scale. In Proceedings of the 2010 International Conference on Information Technology and Scientific Management, Tianjin, China, 20–21 December 2010; pp. 279–282. [Google Scholar]
- Forward, K.M.; Rutledge, G.C. Free surface electrospinning from a wire electrode. Chem. Eng. J. 2012, 183, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.G.; Lee, J.H.; Unnithan, A.R.; Park, C.H.; Kim, C.S. A comprehensive electric field analysis of cylinder-type multi-nozzle electrospinning system for mass production of nanofibers. J. Ind. Eng. Chem. 2015, 31, 251–256. [Google Scholar] [CrossRef]
- Yao, L.R.; Song, X.M.; Zhang, G.Y.; Xu, S.Q.; Jiang, Y.Q.; Cheng, D.H.; Lu, Y.H. Preparation of Ag/HBP/PAN Nanofiber Web and Its Antimicrobial and Filtration Property. J. Nanomater. 2016, 2016, 4515769. [Google Scholar] [CrossRef] [Green Version]
- Khayet, M. Polymeric Nano-Fibers and Modified Nano-Fibers Assembly in 3D Network for Different Potential Applications. J. Mater. Sci. Nanotechnol. 2013, 1, e104. [Google Scholar] [CrossRef] [Green Version]
- Kendouli, S.; Khalfallah, O.; Sobti, N.; Bensouissi, A.; Avci, A.; Eskizeybek, V.; Achour, S. Modification of cellulose acetate nanofibers with PVP/Ag addition. Mater. Sci. Semicond. Process. 2014, 28, 13–19. [Google Scholar] [CrossRef]
- Khalil, K.A.; Fouad, H.; Elsarnagawy, T.; Almajhdi, F.N. Preparation and Characterization of Electrospun PLGA / silver Composite Nanofibers for Biomedical Applications. Int. J. Electrochem. Sci. 2013, 8, 3483–3493. [Google Scholar]
- Kostakova, E.; Meszaros, L.; Gregr, J. Composite nanofibers produced by modified needleless electrospinning. Mater. Lett. 2009, 63, 2419–2422. [Google Scholar] [CrossRef]
- Celebioglu, A.; Aytac, Z.; Umu, O.C.O.; Dana, A.; Tekinay, T.; Uyar, T. One-step synthesis of size-tunable Ag nanoparticles incorporated in electrospun PVA/cyclodextrin nanofibers. Carbohydr. Polym. 2014, 99, 808–816. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Ren, Y.; Zhou, Q.; Chen, J.; Zhi, T.; Lu, Z.; Gao, D.; Ma, Z.; Jin, L. Cotton fabric with plasma pretreatment and ZnO/Carboxymethyl chitosan composite finishing for durable UV resistance and antibacterial property. Carbohydr. Polym. 2016, 138, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Jeon, K.S.; Lim, B.H.; Navamathavan, R.; Kim, H.Y. Preparation and characterization of copper oxide particles incorporated polyurethane composite nanofibers by electrospinning. Ceram. Int. 2013, 39, 9651–9658. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Kanjwal, M.A.; Saran, S.; Chung, W.J.; Kim, H. Polyurethane nanofibers containing copper nanoparticles as future materials. Appl. Surf. Sci. 2011, 257, 3020–3026. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, characterization and antimicrobial activity of copper oxide nanoparticles. J. Nanomater. 2014, 2014, 637858. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nanofiber membranes for antimicrobial breath mask applications. Curr. Res. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Shalaby, T.; Mahmoud, O.; Al-oufy, A. Antibacterial Silver Embedded Nanofibers for Water Disinfection. Int. J. Mater. Sci. Appl. 2015, 4, 293–298. [Google Scholar] [CrossRef]
- Lala, N.L.; Ramaseshan, R.; Bojun, L.; Sundarrajan, S.; Barhate, R.S.; Ying-Jun, L.; Ramakrishna, S. Fabrication of Nanofibers With Antimicrobial Functionality Used as Filters: Protection Against Bacterial Contaminants. Biotechnol. Bioeng. 2007, 97, 1357–1365. [Google Scholar] [CrossRef]
- Cengiz, F.; Jirsak, O. The effect of salt on the roller electrospinning of polyurethane nanofibers. Fibers Polym. 2009, 10, 177–184. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, A.; Mathur, R.B.; Dhakate, S.R. Effective antimicrobial filter from electrospun polyacrylonitrile-silver composite nanofibers membrane for conducive environment. Adv. Mater. Lett. 2014, 5, 562–568. [Google Scholar] [CrossRef]
- Felix Swamidoss, V.; Bangaru, M.; Nalathambi, G.; Sangeetha, D.; Selvam, A.K. Silver-incorporated poly vinylidene fluoride nanofibers for bacterial filtration. Aerosol Sci. Technol. 2019, 53, 196–206. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wu, W.; Zhao, W.; Lan, X. Revealing the antibacterial mechanism of copper surfaces with controllable microstructures. Surf. Coat. Technol. 2020, 395, 125911. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohraz, M.H.; Yu, I.J.; Beitollahi, A.; Dehghan, S.F.; Shin, J.H.; Golbabaei, F. Assessment of the potential release of nanomaterials from electrospun nanofiber filter media. NanoImpact 2020, 19, 100223. [Google Scholar] [CrossRef]














| Solution Properties | 0% | 5% | 7% | 9.5% | 12% |
|---|---|---|---|---|---|
| Conductivity (µS/cm) | 436 | 440 | 450 | 448 | 452 |
| Surface tension (mN/m) | 70.5 | 71.1 | 68.4 | 68.1 | 66.7 |
| Solution Properties | 0% | 5% | 7% | 9.5% | 12% |
|---|---|---|---|---|---|
| Conductivity (µS/cm) | 436 | 447 | 452 | 453 | 451 |
| Surface tension (mN/m) | 70.5 | 70.9 | 66.2 | 67.8 | 67.2 |
| Sample Rotating Needle Cylinder | 95% Confidence | Sample Wire Electrode | 95% Confidence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pristine PU | 182 | 5.4 | 194.5 | 1.07 | Pristine PU | 189 | 5.9 | 199 | 1.05 |
| PU + 5%CuO µm | 226 | 6.2 | 239 | 1.06 | PU + 5%CuO µm | 134 | 4.04 | 142 | 1.06 |
| PU + 5%CuO nm | 228 | 6.04 | 239 | 1.05 | PU + 5%CuO nm | 188 | 6.15 | 202 | 1.07 |
| PU + 7%CuO µm | 278 | 8.5 | 298 | 1.07 | PU + 7%CuO µm | 142 | 3.5 | 149 | 1.05 |
| PU + 7%CuO nm | 262 | 5.97 | 270 | 1.03 | PU + 7%CuO nm | 175 | 5.4 | 186 | 1.06 |
| PU + 9.5%CuO µm | 242 | 6.9 | 257 | 1.06 | PU + 9.5%CuO µm | 125 | 3.2 | 132 | 1.06 |
| PU + 9.5%CuO nm | 237 | 6.1 | 249 | 1.05 | PU + 9.5%CuO nm | 184 | 6.3 | 198 | 1.08 |
| PU + 12%CuO µm | 231 | 5.7 | 249 | 1.08 | PU + 12%CuO µm | 139 | 3.3 | 145 | 1.04 |
| PU + 12%CuO nm | 226 | 6.9 | 240 | 1.06 | PU + 12%CuO nm | 181 | 6.1 | 195 | 1.08 |
| Sample | Surface Density of Produced Nanofibers (g/m²) | |
|---|---|---|
| Rotating Electrode with Needle Surface | Thin Wire Electrode | |
| Pristine PU | 2.5 | 3.43 |
| PU + 5% CuO µm | 12.28 | 4.88 |
| PU + 5% CuO nm | 4.56 | 3.11 |
| PU + 7% CuO µm | 13.05 | 5.31 |
| PU + 7% CuO nm | 9.89 | 4.48 |
| PU + 9.5% CuO µm | 13.93 | 7.63 |
| PU + 9.5% CuO nm | 7.41 | 6.57 |
| PU + 12% CuO µm | 19.46 | 7.25 |
| PU + 12% CuO nm | 5.38 | 6.18 |
| Sample | Efficiency (%)—Escherichia coli | Efficiency (%)—Staphylococcus gallinarum | ||
|---|---|---|---|---|
| µm | nm | µm | nm | |
| PU + 5% CuO | ||||
| cylinder | 97 | 96.8 | 98.8 | 62.7 |
| wire | 64 | 85 | 0 | 17 |
| PU + 7% CuO | ||||
| cylinder | 99.7 | 99.8 | 100 | 96.2 |
| wire | 67 | 90 | 23 | 20 |
| PU + 9.5% CuO | ||||
| cylinder | 100 | 100 | 100 | 98.8 |
| wire | 70 | 89 | 29 | 16 |
| PU + 12% CuO | ||||
| cylinder | 100 | 100 | 100 | 99.6 |
| wire | 81 | 96 | 30 | 30 |
| Sample | Number of Bacteria Passed through the Sample | BFE (%) | Number of Survived Bacteria after the “Smear-Test” |
|---|---|---|---|
| Inoculum | 320 | - | - |
| PU pristine | 17 | 95 | 278 |
| PU + 5% CuO µm | 5 | 98 | 6 |
| PU + 5% CuO nm | 15 | 95 | 13 |
| PU + 7% CuO µm | 0 | 100 | 3 |
| PU + 7% CuO nm | 9 | 97 | 45 |
| PU + 9.5% CuO µm | 0 | 100 | 0 |
| PU + 9.5% CuO nm | 11 | 96.6 | 30 |
| PU + 12% CuO µm | 0 | 100 | 0 |
| PU + 12% CuO nm | 11 | 96.6 | 19 |
| Sample | Number of Bacteria Passed through the Sample | BFE (%) | Number of Survived Bacteria after the “Smear-Test” |
|---|---|---|---|
| Inoculum | 312 | - | - |
| PU pristine | 23 | 93 | 303 |
| PU + 5% CuO µm | 11 | 96 | 14 |
| PU + 5% CuO nm | 13 | 95.8 | 52 |
| PU + 7% CuO µm | 0 | 100 | 2 |
| PU + 7% CuO nm | 30 | 90 | 23 |
| PU + 9.5% CuO µm | 1 | 99.7 | 7 |
| PU + 9.5% CuO nm | 26 | 92 | 22 |
| PU + 12% CuO µm | 0 | 100 | 25 |
| PU + 12% CuO nm | 75 | 76 | 43 |
| Tested Sample | Efficiency (%)— Escherichia coli | Efficiency (%)— Staphylococcus gallinarum | ||
|---|---|---|---|---|
| Before Filtration | After Filtration | Before Filtration | After Filtration | |
| PUR + 5% CuO nm | 96.8 | 86.9 | 62.7 | 30.9 |
| PUR + 7% CuO nm | 99.8 | 91.2 | 98.2 | 80 |
| PUR + 9.5% CuO nm | 100 | 96.8 | 98.8 | 78.3 |
| PUR + 12% CuO nm | 100 | 88.7 | 99.6 | 79.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungur, G.; Hrůza, J. Modified Nanofibrous Filters with Durable Antibacterial Properties. Molecules 2021, 26, 1255. https://doi.org/10.3390/molecules26051255
Ungur G, Hrůza J. Modified Nanofibrous Filters with Durable Antibacterial Properties. Molecules. 2021; 26(5):1255. https://doi.org/10.3390/molecules26051255
Chicago/Turabian StyleUngur, Ganna, and Jakub Hrůza. 2021. "Modified Nanofibrous Filters with Durable Antibacterial Properties" Molecules 26, no. 5: 1255. https://doi.org/10.3390/molecules26051255