Improved Antioxidant Capacity of Black Tea Waste Utilizing PlantCrystals
Abstract
:1. Introduction
2. Results
2.1. Black Tea (BT) Infusion
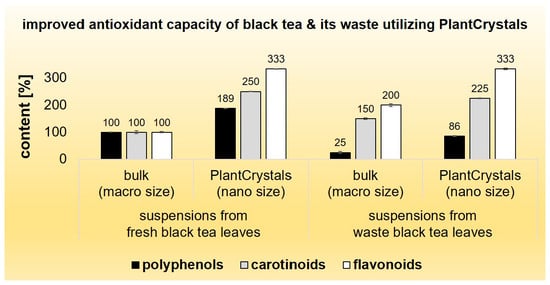
2.2. Bulk-Suspensions from Black Tea (BT) Leaves and Waste
2.3. PlantCrystals from Black Tea (BT) Leaves and Waste
3. Discussion
4. Materials and Methods
4.1. Production of Black Tea Infusion and the PlantCrystals
4.2. PlantCrystal Size Analysis
4.2.1. Light Microscopy (LM)
4.2.2. Laser Diffraction (LD)
4.2.3. Dynamic Light Scattering (DLS)
4.2.4. Zeta-Potential (ZP)
4.3. Total Content and Antioxidant Capacity (AOC) Tests
4.3.1. Total Polyphenol Content (TPC)
4.3.2. Total Flavonoid Content (TFC)
4.3.3. Total Carotenoid Content (TCC)
4.3.4. DPPH● (1,1-diphenyl-2- picrylhydrazyl) Assay
4.3.5. ORAC (Oxygen Radical Absorbance Capacity) Assay
4.3.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Griffin, S.; Sarfraz, M.; Farida, V.; Nasim, M.J.; Ebokaiwe, A.P.; Keck, C.M.; Jacob, C. No time to waste organic waste: Nanosizing converts remains of food processing into refined materials. J. Environ. Manag. 2018, 210, 114–121. [Google Scholar] [CrossRef]
- Sarfraz, M.; Griffin, S.; Gabour Sad, T.; Alhasan, R.; Nasim, M.J.; Irfan Masood, M.; Schäfer, K.H.; Ejike, C.E.C.C.; Keck, C.M.; Jacob, C.; et al. Milling the mistletoe: Nanotechnological conversion of African mistletoe (Loranthus micranthus) intoantimicrobial materials. Antioxidants 2018, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, A.M.; Alnemari, R.M.; Jacob, C.; Keck, C.M. PlantCrystals—Nanosized plant material for improved bioefficacy of medical plants. Materials 2020, 13, 4368. [Google Scholar] [CrossRef]
- Yassin, D.A.; Nasim, M.J.; Abraham, A.M.; Keck, C.M.; Jacob, C. Upcycling culinary organic waste: Production of plant particles from potato and carrot peels to improve antioxidative capacity. Current Nutraceuticals 2020, 1. [Google Scholar] [CrossRef]
- Griffin, S.; Alkhayer, R.; Mirzoyan, S.; Turabyan, A.; Zucca, P.; Sarfraz, M.; Nasim, M.; Trchounian, A.; Rescigno, A.; Keck, C.; et al. Nanosizing Cynomorium: Thumbs up for potential antifungal applications. Inventions 2017, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- All Illustrations Were Created with BioRender. Available online: https://biorender.com/ (accessed on 11 December 2020).
- McKay, D.L.; Blumberg, J.B. Roles for epigallocatechin gallate in cardiovascular disease and obesity: An introduction. J. Am. Coll. Nutr. 2007, 26, 362S–365S. [Google Scholar] [CrossRef]
- Carloni, P.; Tiano, L.; Padella, L.; Bacchetti, T.; Customu, C.; Kay, A.; Damiani, E. Antioxidant activity of white, green and black tea obtained from the same tea cultivar. Food Res. Int. 2013, 53, 900–908. [Google Scholar] [CrossRef]
- Pilgrim, T.S.; Watling, R.J.; Grice, K. Application of trace element and stable isotope signatures to determine the provenance of tea (Camellia sinensis) samples. Food Chem. 2010, 118, 921–926. [Google Scholar] [CrossRef]
- Emekli-Alturfan, E.; Yarat, A.; Akyuz, S. Fluoride levels in various black tea, herbal and fruit infusions consumed in Turkey. Food Chem. Toxicol. 2009, 47, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.A.; Rahman, R.M.A.; Appleton, I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-H.; Chang, H.-H.; Lee, M.-J.; Chen, C.-L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.; de Villiers, W.J.S. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Naoki, N.; Kazumi, K.; Yokota, H.-o. Enhanced lactic acid fermentation of silage by the addition of green tea waste. J. Sci. Food Agric. 2004, 84, 728–734. [Google Scholar] [CrossRef]
- Kondo, M.; Hirano, Y.; Kita, K.; Jayanegara, A.; Yokota, H.-o. Fermentation characteristics, tannin contents and in vitro ruminal degradation of green tea and black tea by-products ensiled at different temperatures. Asian-Australas. J. Anim. Sci. 2014, 27, 937–945. [Google Scholar] [CrossRef]
- Kondo, M.; Kita, K.; Yokota, H.-o. Effects of tea leaf waste of green tea, oolong tea, and black tea addition on sudangrass silage quality andin vitro gas production. J. Sci. Food Agric. 2004, 84, 721–727. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility of gallic acid, catechin, and protocatechuic acid in subcritical water from (298.75 to 415.85) K. J. Chem. Eng. Data 2010, 55, 3101–3108. [Google Scholar] [CrossRef]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Price, W.; Spitzer, J. The temperature dependence of the rate of extraction of soluble constituents of black tea. Food Chem. 1993, 46, 133–136. [Google Scholar] [CrossRef]
- Stahr, P.-L.; Grewal, R.; Eckert, G.P.; Keck, C.M. Investigating hesperetin nanocrystals with tailor-made sizes for the prevention and treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021. accepted manuscript. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Keck, C.M. Flavonoid nanocrystals produced by ARTcrystal®-technology. Int. J. Pharm. 2015, 482, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, I. Alkyl Polyglucosides: From Natural-Origin Surfactants to Prospective Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9781908818775. [Google Scholar]
- Kyle, J.A.M.; Morrice, P.C.; McNeill, G.; Duthie, G.G. Effects of infusion time and addition of milk on content and absorption of polyphenols from black tea. J. Agric. Food Chem. 2007, 55, 4889–4894. [Google Scholar] [CrossRef]
- Pelikh, O.; Stahr, P.-L.; Huang, J.; Gerst, M.; Scholz, P.; Dietrich, H.; Geisel, N.; Keck, C.M. Nanocrystals for improved dermal drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 170–178. [Google Scholar] [CrossRef]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Gohla, S.; Li, L.; Müller, R.H. Long-term stability of quercetin nanocrystals prepared by different methods. J. Pharm. Pharmacol. 2012, 64, 1394–1402. [Google Scholar] [CrossRef]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Bravo, K.; Sepulveda-Ortega, S.; Lara-Guzman, O.; Navas-Arboleda, A.A.; Osorio, E. Influence of cultivar and ripening time on bioactive compounds and antioxidant properties in Cape gooseberry (Physalis peruviana L.). J. Sci. Food Agric. 2015, 95, 1562–1569. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Jiménez, N.; Carrillo-Hormaza, L.; Pujol, A.; Álzate, F.; Osorio, E.; Lara-Guzman, O. Antioxidant capacity and phenolic content of commonly used anti-inflammatory medicinal plants in Colombia. Ind. Crops Prod. 2015, 70, 272–279. [Google Scholar] [CrossRef]










| TPC GAE [mg/g] | TFC QE [mg/g] | TCC β-CE [mg/g] | IC 50 [mg/mL] | ORAC-Value [µmol/µg] | |
|---|---|---|---|---|---|
| Black Tea Infusion | 148 ± 2 | 9 ± 0.2 | 2 ± 0.03 | 0.013 ± 0.005 | 55 ± 2 |
| PlantCrystals | z-Average [nm] | PdI |
|---|---|---|
| BT | 342 ± 7 | 0.4 ± 0.06 |
| BT waste | 279 ± 7 | 0.4 ± 0.02 |
| Sample | TPC GAE [mg/g] | TFC QE [mg/g] | TCC β-CE [mg/g] | IC 50 [mg/mL] | ORAC-Value TE [µmol/µg] |
|---|---|---|---|---|---|
| BT bulk-susp. | 87 ± 0.4 | 9 ± 0.2 | 4 ± 0.2 | 0.051 ± 0.007 | 41 ± 1 |
| BT PlantCrystals | 164 ± 0.4 | 30 ± 0.2 | 10 ± 0.1 | 0.029 ± 0.005 | 40 ± 3 |
| BT waste bulk-susp. | 22 ± 1 | 18 ± 1 | 6 ± 0.2 | 1.157 ± 0.192 | 4 ± 0.3 |
| BT waste PlantCrystals | 75 ± 1 | 30 ± 1 | 9 ± 0.1 | 0.127 ± 0.018 | 14 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abraham, A.M.; Alnemari, R.M.; Brüßler, J.; Keck, C.M. Improved Antioxidant Capacity of Black Tea Waste Utilizing PlantCrystals. Molecules 2021, 26, 592. https://doi.org/10.3390/molecules26030592
Abraham AM, Alnemari RM, Brüßler J, Keck CM. Improved Antioxidant Capacity of Black Tea Waste Utilizing PlantCrystals. Molecules. 2021; 26(3):592. https://doi.org/10.3390/molecules26030592
Chicago/Turabian StyleAbraham, Abraham M., Reem M. Alnemari, Jana Brüßler, and Cornelia M. Keck. 2021. "Improved Antioxidant Capacity of Black Tea Waste Utilizing PlantCrystals" Molecules 26, no. 3: 592. https://doi.org/10.3390/molecules26030592