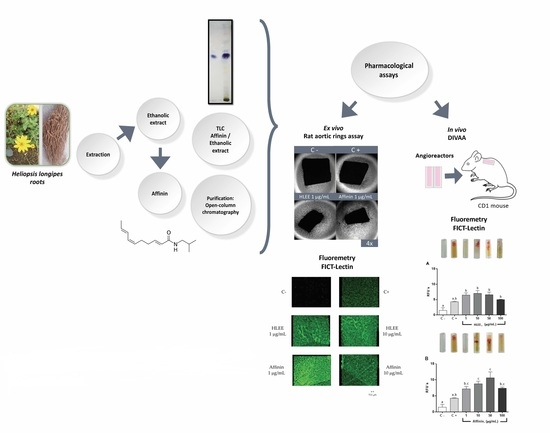
Proangiogenic Effect of Affinin and an Ethanolic Extract from Heliopsis longipes Roots: Ex Vivo and In Vivo Evidence
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Plant Material
4.4. Extraction
4.5. Pharmacological Assays
4.5.1. Rat Aortic Ring Assay
4.5.2. Fluorescence Staining
4.5.3. Direct In Vivo Angiogenesis Assay (DIVAA)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- García-Chávez, A.; Ramírez-Chávez, E.; Molina-Torres, J. El género Heliopsis (Heliantheae; Asteraceae) en México y las alcamidas presentes en sus raíces. Acta Bot. Mex. 2004, 69, 115–131. [Google Scholar] [CrossRef] [Green Version]
- Greger, H. Alkamides: A critical reconsideration of a multifunctional class of unsaturated fatty acid amides. Phytochem. Rev. 2016, 15, 729–770. [Google Scholar] [CrossRef]
- Rios, M.Y. Natural Alkamides: Pharmacology, Chemistry and Distribution. In Drug Discovery Research in Pharmacognosy; Prof. Omboon Vallisuta; InTech; Croatia, 2012; Volume 1, pp. 107–144. [Google Scholar]
- Boonen, J.; Bronselaer, A.; Nielandt, J.; Veryser, L.; De Tré, G.; De Spiegeleer, B. Alkamid database: Chemistry, occurrence and functionality of plant N-alkylamides. J. Ethnopharmacol. 2012, 142, 563–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, M.Y.; Olivo, H.F. Natural and synthetic alkamides: Applications in pain therapy. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 79–121. ISBN 9780444634306. [Google Scholar]
- Ocegueda, S.; Moreno, E.; Koleff, P. Plantas utilizadas en la medicina tradicional y su identificación científica. Biodiversitas 2005, 62, 12–15. [Google Scholar]
- Cilia-López, V.G.; Aguirre-Rivera, J.R.; Espinosa-Reyes, G.; Flores-Cano, J.A.; Reyes-Agüero, J.A.; Juárez-Flores, B.I. Distribución de Heliopsis longipes (Heliantheae: Asteraceae), un recurso endémico del centro de México. Rev. Chapingo Ser. Cienc. For. Ambiente 2014, 20, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Arriaga-Alba, M.; Rios, M.Y.; Déciga-Campos, M. Antimutagenic properties of affinin isolated from Heliopsis longipes extract. Pharm. Biol. 2013, 51, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Martínez, S.; Aguilar-Guadarrama, A.B.; Rios, M.Y. Minor alkamides from Heliopsis longipes S.F. Blake (Asteraceae) fresh roots. Phytochem. Lett. 2011, 4, 275–279. [Google Scholar] [CrossRef]
- Silveira, N.; Sandjo, L.P.; Biavatti, M.W. Spilanthol-containing products: A patent review (1996–2016). Trends Food Sci. Technol. 2018, 74, 107–111. [Google Scholar] [CrossRef]
- Boonen, J.; Baert, B.; Burvenich, C.; Blondeel, P.; De Saeger, S.; De Spiegeleer, B. LC–MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J. Pharm. Biomed. Anal. 2010, 53, 243–249. [Google Scholar] [CrossRef]
- Correa, J.; Roquet, S.; Díaz, E. Multiple NMR analysis of the affinin. Org. Magn. Reson. 1971, 3, 1–5. [Google Scholar] [CrossRef]
- Johns, T.; Graham, K.; Towers, G.H.N. Molluscicidal activity of affinin and other isobutylamides from the asteraceae. Phytochemistry 1982, 21, 2737–2738. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Boonen, J.; Chauhan, N.S.; Thakur, M.; De Spiegeleer, B.; Dixit, V.K. Spilanthes acmella ethanolic flower extract: LC–MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine 2011, 18, 1161–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Torres, J.; Salgado-Garciglia, R.; Ramírez-Chávez, E.; Del Río, R.E. Purely Olefinic Alkamides in Heliopsis longipes and Acmella (Spilanthes) oppositifolia. Biochem. Syst. Ecol. 1996, 24, 43–47. [Google Scholar] [CrossRef]
- Hernández-Morales, A.; Arvizu-Gómez, J.L.; Carranza-Álvarez, C.; Gómez-Luna, B.E.; Alvarado-Sánchez, B.; Ramírez-Chávez, E.; Molina-Torres, J. Larvicidal activity of affinin and its derived amides from Heliopsis longipes A. Gray Blake against Anopheles albimanus and Aedes aegypti. J. Asia. Pac. Entomol. 2015, 18, 227–231. [Google Scholar] [CrossRef]
- Molina-Torres, J.; García-Chávez, A.; Ramírez-Chávez, E. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J. Ethnopharmacol. 1999, 64, 241–248. [Google Scholar] [CrossRef]
- Molina-Torres, J.; Salazar-Cabrera, C.J.; Armenta-Salinas, C.; Ramírez-Chávez, E. Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: Affinin and reduced amides. J. Agric. Food Chem. 2004, 52, 4700–4704. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Fan, N.C.; Lin, M.H.; Chu, I.R.; Huang, S.J.; Hu, C.Y.; Han, S.Y. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef]
- Hernández, I.; Márquez, L.; Martínez, I.; Dieguez, R.; Delporte, C.; Prieto, S.; Molina-Torres, J.; Garrido, G. Anti-inflammatory effects of ethanolic extract and alkamides-derived from Heliopsis longipes roots. J. Ethnopharmacol. 2009, 124, 649–652. [Google Scholar] [CrossRef]
- Cariño-Cortés, R.; Gayosso-De-Lucio, J.A.; Ortiz, M.I.; Sánchez-Gutiérrez, M.; García-Reyna, P.B.; Cilia-López, V.G.; Pérez-Hernández, N.; Moreno, E.; Ponce-Monter, H. Antinociceptive, genotoxic and histopathological study of Heliopsis longipes S.F. Blake in mice. J. Ethnopharmacol. 2010, 130, 216–221. [Google Scholar] [CrossRef]
- Déciga-Campos, M.; Rios, M.Y.; Aguilar-Guadarrama, A.B. Antinociceptive effect of Heliopsis longipes extract and affinin in mice. Planta Med. 2010, 76, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Gerbino, A.; Schena, G.; Milano, S.; Milella, L.; Barbosa, A.F.; Armentano, F.; Procino, G.; Svelto, M.; Carmosino, M. Spilanthol from Acmella oleracea lowers the intracellular levels of cAMP impairing NKCC2 phosphorylation and water channel AQP2 membrane expression in mouse kidney. PLoS ONE 2016, 11, e0156021. [Google Scholar] [CrossRef]
- Acosta-Madrid, I.I.; Castañeda-Hernández, G.; Cilia-López, V.G.; Cariño-Cortés, R.; Pérez-Hernández, N.; Fernández-Martínez, E.; Ortiz, M.I. Interaction between Heliopsis longipes extract and diclofenac on the thermal hyperalgesia test. Phytomedicine 2009, 16, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Arriaga-Alba, M.; Ventura-Martínez, R.; Aguilar-Guadarrama, B.; Rios, M.Y. Pharmacological and toxicological profile of extract from Heliopsis longipes and affinin. Drug Dev. Res. 2012, 73, 130–137. [Google Scholar] [CrossRef]
- De la Rosa-Lugo, V.; Acevedo-Quiroz, M.; Déciga-Campos, M.; Rios, M.Y. Antinociceptive effect of natural and synthetic alkamides involves TRPV1 receptors. J. Pharm. Pharmacol. 2017, 69, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Baert, B.; Roche, N.; Burvenich, C.; De Spiegeleer, B. Transdermal behaviour of the N-alkylamide spilanthol (affinin) from Spilanthes acmella (Compositae) extracts. J. Ethnopharmacol. 2010, 127, 77–84. [Google Scholar] [CrossRef]
- Veryser, L.; Taevernier, L.; Joshi, T.; Tatke, P.; Wynendaele, E.; Bracke, N.; Stalmans, S.; Peremans, K.; Burvenich, C.; Risseeuw, M.; et al. Mucosal and blood-brain barrier transport kinetics of the plant N-alkylamide spilanthol using in vitro and in vivo models. BMC Complement. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Ruiz, J.E.; Rojas-Molina, A.; Luna-Vázquez, F.J.; Rivero-Cruz, F.; García-Gasca, T.; Ibarra-Alvarado, C. Affinin (Spilanthol), Isolated from Heliopsis longipes, Induces Vasodilation via Activation of Gasotransmitters and Prostacyclin Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 218. [Google Scholar] [CrossRef]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.-W.; Li, H.; Guedez, L.; Wingfield, P.T.; Diaz, T.; Salloum, R.; Wei, B.; Stetler-Stevenson, W.G. TIMP-2 mediated inhibition of angiogenesis: An MMP-independent mechanism. Cell 2003, 114, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Ward, Y.; Tian, L.; Lake, R.; Guedez, L.; Stetler-Stevenson, W.G.; Kelly, K. CD97, an adhesion receptor on inflammatory cells, stimulates angiogenesis through binding integrin counterreceptors on endothelial cells. Blood 2005, 105, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Wong, W. Two Gases Required for Vasodilation and Angiogenesis. Sci. Signal. 2012, 5, ec163. [Google Scholar] [CrossRef]
- Valencia-Guzmán, C.J.; Castro-Ruiz, J.E.; García-Gasca, T.; Rojas-Molina, A.; Romo-Mancillas, A.; Luna-Vázquez, F.J.; Rojas-Molina, J.I.; Ibarra-Alvarado, C. Endothelial TRP channels and cannabinoid receptors are involved in affinin-induced vasodilation. Fitoterapia 2021, 153, 104985. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, H.; Jiang, Y.; Yuan, Y.; Zhang, Q.; Guo, Q.; Gong, P. CGRP regulates the dysfunction of peri-implant angiogenesis and osseointegration in streptozotocin-induced diabetic rats. Bone 2020, 139, 115464. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Xu, J.; Yao, H.; Li, X.; Tong, W.; Li, Y.; Dai, B.; He, X.; Chow, D.H.K.; Li, G.; et al. Calcitonin Gene-Related Peptide Enhances Distraction Osteogenesis by Increasing Angiogenesis. Tissue Eng.—Part A 2021, 27, 87–102. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Katsouda, A.; Bibli, S.-I.; Pyriochou, A.; Szabo, C.; Papapetropoulos, A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol. Res. 2016, 113, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Szabó, C.; Papapetropoulos, A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br. J. Pharmacol. 2011, 164, 853–865. [Google Scholar] [CrossRef] [Green Version]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Cilia-López, V.G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Reyes-Agüero, J.A. Analgesic activity of Heliopsis longipes and its effect on the nervous system. Pharm. Biol. 2010, 48, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; Aguilar-Guadarrama, A.B.; Gutiérrez, M.D.C. Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae). J. Ethnopharmacol. 2007, 110, 364–367. [Google Scholar] [CrossRef]
- Go, R.S.; Ritman, E.L.; Owen, W.G. Angiogenesis in rat aortic rings stimulated by very low concentrations of serum and plasma. Angiogenesis 2003, 6, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Zippel, N.; Ding, Y.; Fleming, I. A modified aortic ring assay to assess angiogenic potential in vitro. Methods Mol. Biol. 2016, 1430, 205–219. [Google Scholar] [PubMed]
- Aplin, A.C.; Nicosia, R.F. The rat aortic ring model of angiogenesis. In Vascular Morphogenesis: Methods and Protocols, 1st ed.; Domenico Ribatti; Humana Press: Clifton, NJ, USA, 2015; pp. 255–264. ISBN 9781493914623. [Google Scholar]
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and assays for the study of angiogenesis. Exp. Biol. Med. (Maywood) 2014, 239, 1476–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedez, L.; Rivera, A.M.; Salloum, R.; Miller, M.L.; Diegmueller, J.J.; Bungay, P.M.; Stetler-Stevenson, W.G. Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am. J. Pathol. 2003, 162, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of Angiogenesis Assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinda, K.M. In vivo matrigel migration and angiogenesis assay. Methods Mol. Biol. 2009, 467, 287–294. [Google Scholar] [CrossRef]
- Staton, C.A.; Stribbling, S.M.; Tazzyman, S.; Hughes, R.; Brown, N.J.; Lewis, C.E. Current methods for assaying angiogenesis in vitro and in vivo. Int. J. Exp. Pathol. 2004, 85, 233–248. [Google Scholar] [CrossRef]
- Min, J.-K.; Han, K.-Y.; Kim, E.-C.; Kim, Y.-M.; Lee, S.-W.; Kim, O.-H.; Kim, K.-W.; Gho, Y.S.; Kwon, Y.-G. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004, 64, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, F.; Feng, L.; Cai, W.; Qiu, Y.; Liu, Y.; Li, Y.; Du, B.; Qiu, L. Vaccarin promotes endothelial cell proliferation in association with neovascularization in vitro and in vivo. Mol. Med. Rep. 2015, 12, 1131–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zihlif, M.; Afifi, F.; Muhtaseb, R.; Al-Khatib, S.; Abaza, I.; Naffa, R. Screening the Antiangiogenic Activity of Medicinal Plants Grown and Sold in Jordan. Planta Med. 2012, 78, 297–301. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, A.; Chen, C.G.; Iozzo, R.V. A simplified aortic ring assay: A useful ex vivo method to assess biochemical and functional parameters of angiogenesis. Matrix Biol. Plus 2020, 6–7, 100025. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem. J. 1987, 19, 225–234. [Google Scholar] [CrossRef]
- Baker, M.; Robinson, S.D.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’Amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Blacher, S.; Devy, L.; Burbridge, M.F.; Roland, G.; Tucker, G.; Noël, A.; Foidart, J.M. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis 2001, 4, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Yoneda, J.; Bucana, C.D.; Fidler, I.J. Regulation of distinct steps of angiogenesis by different angiogenic molecules. Int. J. Oncol. 1998, 12, 749–757. [Google Scholar] [CrossRef]
- Wasiutyński, A.; Bałan, B.J.; Skopińska-Rózewska, E.; Siwicki, A.K.; Skurzak, H.; Chorostowska-Wynimko, J.; Sommer, E.; Mazurkiewicz, M. The effect of Echinacea purpurea on the morphology, angiogenic activity and vascular endothelial growth factor (VEGF) concentration of murine L-1 sarcoma tumors. Cent. J. Immunol. 2009, 34, 38–41. [Google Scholar]
- Rogala, E.; Skopińska-Rózewska, E.; Sommer, E.; Pastewka, K.; Chorostowska-Wynimko, J.; Sokolnicka, I.; Kazoń, M. Assessment of the VEGF, bFGF, aFGF and IL8 angiogenic activity in urinary bladder carcinoma, using the mice cutaneous angiogenesis test. Anticancer Res. 2001, 21, 4259–4263. [Google Scholar] [PubMed]
- Nomura, E.C.O.; Rodrigues, M.R.A.; da Silva, C.F.; Hamm, L.A.; Nascimento, A.M.; de Souza, L.M.; Cipriani, T.R.; Baggio, C.H.; de Werner, M.F.P. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J. Ethnopharmacol. 2013, 150, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.A.; Barth, S.; Waldeck-Weiermair, M.; Klec, C.; Strunk, D.; Malli, R.; Graier, W.F. TRPV1 mediates cellular uptake of anandamide and thus promotes endothelial cell proliferation and network-formation. Biol. Open 2014, 3, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Pisanti, S.; Borselli, C.; Oliviero, O.; Laezza, C.; Gazzerro, P.; Bifulco, M. Antiangiogenic activity of the endocannabinoid anandamide: Correlation to its tumor-suppressor efficacy. J. Cell. Physiol. 2007, 211, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Herradón, E.; Martín, M.I.; López-Miranda, V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br. J. Pharmacol. 2007, 152, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picardi, P.; Ciaglia, E.; Proto, M.; Pisanti, S. Anandamide inhibits breast tumor-induced angiogenesis. Transl. Med. @ UniSa 2014, 10, 8–12. [Google Scholar] [CrossRef]
- Yamane, L.T.; de Paula, E.; Jorge, M.P.; de Freitas-Blanco, V.S.; Junior, Í.M.; Figueira, G.M.; Anholeto, L.A.; de Oliveira, P.R.; Rodrigues, R.A.F. Acmella oleracea and Achyrocline satureioides as Sources of Natural Products in Topical Wound Care. Evid. Based. Complement. Alternat. Med. 2016, 2016, 3606820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dermane, F.; Passaro, G. Use of an Acmella oleracea extract for the botulinum toxin-like effect thereof in an anti-wrinkle cosmetic composition. Google Patents. U.S. Patent 7,531,193, 12 May 2009. [Google Scholar]
- Veryser, L.; Bracke, N.; Wynendaele, E.; Joshi, T.; Tatke, P.; Taevernier, L.; De Spiegeleer, B. Quantitative in Vitro and in Vivo Evaluation of Intestinal and Blood-Brain Barrier Transport Kinetics of the Plant N -Alkylamide Pellitorine. Biomed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veryser, L.; Wynendaele, E.; Taevernier, L.; Verbeke, F.; Joshi, T.; Tatke, P.; De Spiegeleer, B. N-alkylamides: From plant to brain. Funct. Foods Heal. Dis. 2014, 4, 264–275. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; De La Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Trejo, B.; Morales-Hernández, M.N.; González-Anduaga, G.M.; Balderas-López, J.L.; Tavares-Carvalho, J.C.; Navarrete, A. Evidence for Involvement of TRPV1 Receptors and Potassium Channels in the Seizures Induced by α-Sanshool. Planta Medica Int. Open 2019, 6, e23–e27. [Google Scholar] [CrossRef] [Green Version]
- Lieder, B.; Zaunschirm, M.; Holik, A.-K.; Ley, J.P.; Hans, J.; Krammer, G.E.; Somoza, V. The Alkamidetrans-Pellitorine Targets PPARγ via TRPV1 and TRPA1 to Reduce Lipid Accumulation in Developing 3T3-L1 Adipocytes. Front. Pharmacol. 2017, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohm, B.; Riedel, A.; Ley, J.P.; Widder, S.; Krammer, G.E.; Somoza, V. Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme A synthetase activity in Caco-2 cells. Food Funct. 2015, 6, 172–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanaga, A.; Goto, H.; Nakagawa, T.; Hikiami, H.; Shibahara, N.; Shimada, Y. Cinnamaldehyde Induces Endothelium-Dependent and -Independent Vasorelaxant Action on Isolated Rat Aorta. Biol. Pharm. Bull. 2006, 29, 2415–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earley, S.; Brayden, J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpure, B.V.; Bian, J.-S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell. Longev. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bełtowski, J.; Jamroz-Wisniewska, A. Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules 2014, 19, 21506–21528. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana, NOM-062-ZOO-1999, Especificaciones Técnicas Para la Producción, Cuidado y uso de los Animales de Laboratorio. Available online: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 16 December 2021).
- Willig, J.B.; Salomón, J.L.D.O.; Vianna, D.R.B.; Moura, S.; Pilger, D.A.; Buffon, A.; Konrath, E.L. Heliopsis longipes S.F. Blake (Asteraceae) extract causes cell cycle arrest and induces caspase dependent apoptosis against cancer cell lines. S. Afr. J. Bot. 2019, 125, 251–260. [Google Scholar] [CrossRef]
- Martinez-Loredo, E.; Izquierdo-Vega, J.A.; Cariñ O-Cortes, R.; Cilia-Ló Pez, V.G.; Madrigal-Santillán, E.O.; Zuñ Iga-Pérez, C.; Valadez-Vega, C.; Moreno, E.; Sánchez-Gutiérrez, M. Effects of Heliopsis longipes ethanolic extract on mouse spermatozoa in vitro. Pezzuto Pharm Biol 2016, 54, 266–271. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Badillo, P.E.; Avalos-Soriano, A.; López-Martínez, J.; García-Gasca, T.; Castro-Ruiz, J.E. Proangiogenic Effect of Affinin and an Ethanolic Extract from Heliopsis longipes Roots: Ex Vivo and In Vivo Evidence. Molecules 2021, 26, 7670. https://doi.org/10.3390/molecules26247670
García-Badillo PE, Avalos-Soriano A, López-Martínez J, García-Gasca T, Castro-Ruiz JE. Proangiogenic Effect of Affinin and an Ethanolic Extract from Heliopsis longipes Roots: Ex Vivo and In Vivo Evidence. Molecules. 2021; 26(24):7670. https://doi.org/10.3390/molecules26247670
Chicago/Turabian StyleGarcía-Badillo, Paola Estefanía, Anaguiven Avalos-Soriano, Josué López-Martínez, Teresa García-Gasca, and Jesús Eduardo Castro-Ruiz. 2021. "Proangiogenic Effect of Affinin and an Ethanolic Extract from Heliopsis longipes Roots: Ex Vivo and In Vivo Evidence" Molecules 26, no. 24: 7670. https://doi.org/10.3390/molecules26247670