Synthesis of Fe2+ Substituted High-Performance LiMn1−xFexPO4/C (x = 0, 0.1, 0.2, 0.3, 0.4) Cathode Materials for Lithium-Ion Batteries via Sol-Gel Processes
Abstract
:1. Introduction
2. Result and Discussion
2.1. Phase Structure Analysis
2.2. Micromorphology Analysis
2.3. Electrochemical Analysis
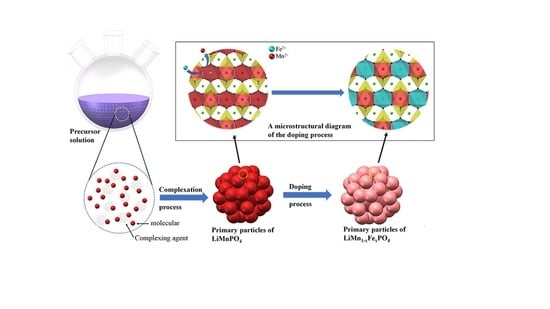
3. Experimental Section
3.1. Material and Methods
3.2. Characterization of Synthesized Materials
3.3. Electrode Preparation and Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jing, L.; Chaowei, G.; Yuanbin, Q.; Xiaohui, N. Ascorbic acid-assisted solvothermal synthesis of LiMn1-xFexPO4/C nanoparticles for high-performance Li-ion cathode materials. Mater. Technol. 2020, 35, 565–571. [Google Scholar]
- Sana, D.; Benoît, M. Characterization of carbon-coated LiFe0.3Mn0.7PO4 particles produced using a colloidal method for lithium batteries. J. Alloys Compd. 2018, 766, 1067–1073. [Google Scholar]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.S.; Madhavi, S. LiMnPO4—A next generation cathode material for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 3518–3539. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, C.R.; Ahn, J.H.; Matic, A.; Jacobsson, P. Highly porous LiMnPO4 in combination with an ionic liquid-based polymer gel electrolyte for lithium batteries. Electrochem. Commun. 2011, 13, 1105–1108. [Google Scholar] [CrossRef]
- Liu, H.; Ren, L.; Li, J.; Tuo, H. Iron-assisted carbon coating strategy for improved electrochemical LiMn0.8Fe0.2PO4 cathodes. Electrochim. Acta 2016, 212, 800–807. [Google Scholar] [CrossRef]
- Memm, M.; Koentje, M.; Axmann, P.; Wohlfahrt-Mehrens, M. New high-voltage step at 4.8 V in cobalt free manganese based lithium phospho olivines for lithium-ion batteries. J. Power Sources 2015, 276, 382–387. [Google Scholar] [CrossRef]
- Liu, J.; Bi, Y.; Yang, W.; Liu, M.; Ren, Z.; Zhang, S.; Wang, D. Re-considering the LiMn1-xFexPO4/C cathodes utilized in electric vehicles. Ionics 2020, 26, 3215–3221. [Google Scholar] [CrossRef]
- Haghi, Z.; Masoudpanah, S.M. CTAB-assisted solution combustion synthesis of LiFePO4 powders. J. Solgel Sci. Technol. 2019, 91, 335–341. [Google Scholar] [CrossRef]
- Li, S.; Su, Z.; Muslim, A.; Jiang, X.; Wang, X. Preparation and electrochemical properties of LiMn1−xFexPO4/C composite cathode materials for lithium ion batteries. Ceram. Int. 2015, 41, 11132–11135. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Qiao, J.; Chang, C. High performance of LiMn1-xFexPO4/C (0 ≤ x ≤ 0.5) nanoparticles synthesized by microwave-assisted solvothermal method. Ionics 2017, 24, 689–696. [Google Scholar] [CrossRef]
- Syahrial, A.Z.; Pawito, W.C.; Sofyan, N.; Rahmatulloh, W.M.F. Performance of Vanadium Doped and Carbon Bamboo/Carbon Black Coated Lithium Iron Phosphate for Battery Cathode. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012023. [Google Scholar] [CrossRef]
- Xue, Z.; Li, L.; Cao, L.; Zheng, W.; Yang, W.; Yu, X. A simple method to fabricate NiFe2O4/NiO@Fe2O3 core-shelled nanocubes based on Prussian blue analogues for lithium ion battery. J. Alloys Compd. 2020, 825, 153966. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, C.; Liu, J.; Bian, P.; Hao, H.; Yu, A. Enhancing the performance of LiMnPO4/C composites through Cr doping. J. Alloys Compd. 2015, 620, 350–357. [Google Scholar] [CrossRef]
- Liu, L.; Chen, G.; Du, B.; Cui, Y.; Ke, X.; Liu, J.; Guo, Z.; Shi, Z.; Zhang, H.; Chou, S. Nano-sized cathode material LiMn0.5Fe0.5PO4/C synthesized via improved sol-gel routine and its magnetic and electrochemical properties. Electrochim. Acta 2017, 255, 205–211. [Google Scholar] [CrossRef]
- Moskon, J.; Pivko, M.; Jerman, I.; Tchernychova, E.; Logar, N.Z.; Zorko, M.; Selih, V.S.; Dominko, R.; Gaberscek, M. Cycling stability and degradation mechanism of LiMnPO4 based electrodes. J. Power Sources 2016, 303, 97–108. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, R.; Yang, X. Enhanced electrochemical properties of LiFePO4/C synthesized with two kinds of carbon sources, PEG-4000 (organic) and Super p (inorganic). Ceram. Int. 2014, 40, 8439–8444. [Google Scholar] [CrossRef]
- Xiang, W.; Wu, Z.G.; Wang, E.H.; Chen, M.Z.; Song, Y.; Zhang, J.B.; Zhong, Y.J.; Chou, S.L.; Luo, J.H.; Guo, X.D. Confined synthesis of graphene wrapped LiMn0.5Fe0.5PO4 composite via two step solution phase method as high performance cathode for Li-ion batteries. J. Power Sources 2016, 329, 94–103. [Google Scholar] [CrossRef]
- Hong, J.; Wang, F.; Wang, X.; Graetz, J. LiFexMn1−xPO4: A cathode for lithium-ion batteries. J. Power Sources 2011, 196, 3659–3663. [Google Scholar] [CrossRef]
- Ding, J.; Su, Z.; Tian, H. Synthesis of high rate performance LiFe1−xMnxPO4/C composites for lithium-ion batteries. Ceram. Int. 2016, 42, 12435–12440. [Google Scholar] [CrossRef]
- Li, B.Z.; Wang, Y.; Xue, L.; Li, X.P.; Li, W.S. Acetylene black-embedded LiMn0.8Fe0.2PO4/C composite as cathode for lithium ion battery. J. Power Sources 2013, 232, 12–16. [Google Scholar] [CrossRef]
- Li, Z.; Dong, G.; Kang, J.; Li, L. Preparation and electrochemical properties of nanoparticle structural LiFePO4/C by Sol–Gel method as cathode material for lithium ion batteries. J. Mater. Sci. Mater. Electron. 2019, 30, 6593–6600. [Google Scholar] [CrossRef]
- Yu, J.; Zhan, H.H.; Hao, W.J. Construction of the nano-carbon connections among the carbon-coated LiFe0.8Mn0.2PO4 grains by sol-gel technique. Russ. J. Electrochem. 2014, 50, 345–349. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Du, K.; Peng, Z.; Xie, X.; Cao, Y. Synthesis and characterization of LiMn0.8Fe0.2PO4/rGO/C for lithium-ion batteries via in-situ coating of Mn0.8Fe0.2C2O4·2H2O precursor with graphene oxide. J. Solid State Electrochem. 2020, 24, 2441–2450. [Google Scholar] [CrossRef]
- Mei, R.; Yang, Y.; Song, X.; An, Z.; Yan, K.; Zhang, J. Graphene encapsulated spherical hierarchical superstructures self-assembled by LiFe0.75Mn0.25PO4 nanoplates for high-performance Li-ion batteries. Electrochim. Acta 2016, 218, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Wen, F.; Lv, T.; Gao, P.; Wu, B.; Liang, Q.; Zhang, Y.; Shu, H.; Yang, X.; Liu, L.; Wang, X. Graphene-embedded LiMn0.8Fe0.2PO4 composites with promoted electrochemical performance for lithium ion batteries. Electrochim. Acta 2018, 276, 134–141. [Google Scholar] [CrossRef]
- Hamid, N.A.; Wennig, S.; Heinzel, A.; Schulz, C.; Wiggers, H. Influence of carbon content, particle size, and partial manganese substitution on the electrochemical performance of LiFexMn1-xPO4/carbon composites. Ionics 2015, 21, 1857–1866. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Wang, Y.; Li, Z.; Zhang, J. Three-dimensional (3D) LiMn0.8Fe0.2PO4 nanoflowers assembled from interconnected nanoflakes as cathode materials for lithium ion batteries. Ceram. Int. 2017, 43, 3190–3195. [Google Scholar] [CrossRef]
- Fang, H.; Pan, Z.; Li, L.; Yang, Y.; Yan, G.; Li, G.; Wei, S. The possibility of manganese disorder in LiMnPO4 and its effect on the electrochemical activity. Electrochem. Commun. 2008, 10, 1071–1073. [Google Scholar] [CrossRef]
- Hong, Y.; Tang, Z.; Hong, Z.; Zhang, Z. LiMn1−xFexPO4 (x = 0, 0.1, 0.2) nanorods synthesized by a facile solvothermal approach as high performance cathode materials for lithium-ion batteries. J. Power Sources 2014, 248, 655–659. [Google Scholar] [CrossRef]
- Kobayashi, G.; Yamada, A.; Nishimura, S.-I.; Kanno, R.; Kobayashi, Y.; Seki, S.; Ohno, Y.; Miyashiro, H. Shift of redox potential and kinetics in Lix(MnyFe1−y)PO4. J. Power Sources 2009, 189, 397–401. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, P.; Yao, Q. Electrochemical performance of LiFePO4/C synthesized by sol-gel method as cathode for aqueous lithium ion batteries. J. Alloys Compd. 2018, 741, 404–408. [Google Scholar] [CrossRef]
- Di Lecce, D.; Hu, T.; Hassoun, J. Electrochemical features of LiMnPO4 olivine prepared by sol-gel pathway. J. Alloys Compd. 2017, 693, 730–737. [Google Scholar] [CrossRef]
- Laveda, J.V.; Johnston, B.; Paterson, G.W.; Baker, P.J.; Tucker, M.G.; Playford, H.Y.; Jensen, K.M.Ø.; Billinge, S.J.L.; Corr, S.A. Structure–property insights into nanostructured electrodes for Li-ion batteries from local structural and diffusional probes. J. Mater. Chem. A 2018, 6, 127–137. [Google Scholar] [CrossRef] [Green Version]
- El Khalfaouy, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Synthesis and characterization of Na-substituted LiMnPO4 as a cathode material for improved lithium ion batteries. J. Alloys Compd. 2019, 775, 836–844. [Google Scholar] [CrossRef]
- Esmezjan, L.; Mikhailova, D.; Etter, M.; Cabana, J.; Grey, C.P.; Indris, S.; Ehrenberg, H. Electrochemical Lithium Extraction and Insertion Process of Sol-Gel Synthesized LiMnPO4 via Two-Phase Mechanism. J. Electrochem. Soc. 2019, 166, A1257–A1265. [Google Scholar] [CrossRef]








| Sample | a (Å) | b (Å) | c (Å) | v (Å)3 |
|---|---|---|---|---|
| LiMnPO4/C | 10.445 | 6.102 | 4.745 | 302.61 |
| LiMn0.9Fe0.1PO4/C | 10.434 | 6.088 | 4.741 | 301.22 |
| LiMn0.8Fe0.2PO4/C | 10.425 | 6.081 | 4.737 | 300. 7 |
| LiMn0.7Fe0.3PO4/C | 10.412 | 6.073 | 4.732 | 299.12 |
| LiMn0.6Fe0.4PO4/C | 10.405 | 6.065 | 4.727 | 298.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, K.; Zhu, J.; Xie, Q.; Men, Y.; Yang, W.; Li, J.; Yu, X. Synthesis of Fe2+ Substituted High-Performance LiMn1−xFexPO4/C (x = 0, 0.1, 0.2, 0.3, 0.4) Cathode Materials for Lithium-Ion Batteries via Sol-Gel Processes. Molecules 2021, 26, 7641. https://doi.org/10.3390/molecules26247641
Fang K, Zhu J, Xie Q, Men Y, Yang W, Li J, Yu X. Synthesis of Fe2+ Substituted High-Performance LiMn1−xFexPO4/C (x = 0, 0.1, 0.2, 0.3, 0.4) Cathode Materials for Lithium-Ion Batteries via Sol-Gel Processes. Molecules. 2021; 26(24):7641. https://doi.org/10.3390/molecules26247641
Chicago/Turabian StyleFang, Kaibin, Jihua Zhu, Qian Xie, Yifei Men, Wei Yang, Junpeng Li, and Xinwei Yu. 2021. "Synthesis of Fe2+ Substituted High-Performance LiMn1−xFexPO4/C (x = 0, 0.1, 0.2, 0.3, 0.4) Cathode Materials for Lithium-Ion Batteries via Sol-Gel Processes" Molecules 26, no. 24: 7641. https://doi.org/10.3390/molecules26247641