New Unsymmetrically Substituted Benzothiadiazole-Based Luminophores: Synthesis, Optical, Electrochemical Studies, Charge Transport, and Electroluminescent Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Optical Properties
2.3. The Charge Transport Study
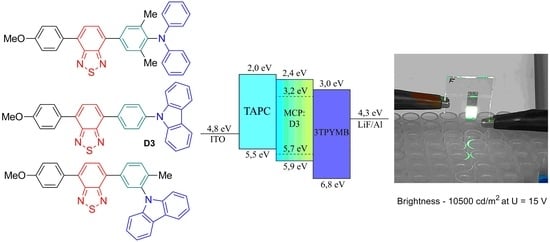
2.4. OLED Device Fabrication and Study of Their Spectral and Optoelectronic Properties
3. Materials and Methods
3.1. General Information
3.2. Preparation and Characterization of Novel Compounds
3.2.1. 9-(5-Bromo-2-methylphenyl)-9H-carbazole (3)
3.2.2. 9-(2-Methyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole (4)
3.2.3. General Procedure for the Suzuki Synthesis of 2, D2 and D3
3.2.4. 4-(7-(4-Methoxyphenyl)benzo[c][1,2,5]thiadiazol-4-yl)-2,6-dimethylaniline (2)
3.2.5. 4-(7-(4-Methoxyphenyl)benzo[c][1,2,5]thiadiazol-4-yl)-2,6-dimethyl-N,N-diphenylaniline (D1)
3.2.6. 4-(3-(9H-Carbazol-9-yl)-4-methylphenyl)-7-(4-methoxyphenyl)benzo[c][1,2,5]thiadiazole (D2)
3.2.7. 4-(4-(9H-Carbazol-9-yl)phenyl)-7-(4-methoxyphenyl)benzo[c][1,2,5]thiadiazole (D3)
3.3. Absorption and Emission Spectra, Photoluminescence Quantum Yield
3.4. Photoluminescence Decay Measurements
3.5. Voltammetry Studies
3.6. Charge Mobility Measurements
3.7. OLED Device Fabrication and Characterization
3.8. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Pisula, W.; Tsao, H.N.; Ellinger, S.; Mullen, K.; Reynolds, J.R. Tailoring Structure-Property Relationships in Dithienosilole-Benzothiadiazole Donor-Acceptor Copolymers. J. Am. Chem. Soc. 2009, 131, 7514–7515. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A Low-Bandgap Poly(2,7-carbazole) Derivative for Use in High-Performance Solar Cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Y. Conjugated Polymer Photovoltaic Materials with Broad Absorption Band and High Charge Carrier Mobility. Adv. Mater. 2008, 20, 2952–2958. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly Efficient Solar Cell Polymers Developed via Fine-Tuning of Structural and Electronic Properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupre, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk Heterojunction Solar Cells with Internal Quantum Efficiency Approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Hau, S.K.; Yip, H.-L.; Davies, J.A.; Chen, K.-S.; Zhang, Y.; Sun, Y.; Jen, A.K.-Y. Benzobis(silolothiophene)-Based Low Bandgap Polymers for Efficient Polymer Solar Cells. Chem. Mater. 2011, 23, 765–767. [Google Scholar] [CrossRef]
- Zhang, Y.; Hau, S.K.; Yip, H.-L.; Sun, Y.; Acton, O.; Jen, A.K.-Y. Efficient Polymer Solar Cells Based on the Copolymers of Benzodithiophene and Thienopyrroledione. Chem. Mater. 2010, 22, 2696–2698. [Google Scholar] [CrossRef]
- Zou, Y.; Najari, A.; Berrouard, P.; Beaupre, S.; Aïch, B.R.; Tao, Y.; Leclerc, M. A Thieno[3,4-c]pyrrole-4,6-dione-Based Copolymer for Efficient Solar Cells. J. Am. Chem. Soc. 2010, 132, 5330–5331. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Champion, R.D.; Jenekhe, S.A. Conjugated Donor-Acceptor Copolymer Semiconductors with Large Intramolecular Charge Transfer: Synthesis, Optical Properties, Electrochemistry, and Field Effect Carrier Mobility of Thienopyrazine-Based Copolymers. Macromolecules 2006, 39, 8712–8719. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Tsagkournos, D.V.; Sharma, S.S.; Vijay, Y.K.; Sharma, G.D. Low Bandgap Conjugated Small Molecules Containing Benzobisthiadiazole and Thienothiadiazole Central Units: Synthesis and Application for Bulk Heterojunction Solar Cells. J. Mater. Chem. 2011, 21, 4679–4688. [Google Scholar] [CrossRef]
- Zheng, Q.; Jung, B.J.; Sun, J.; Katz, H.E. Ladder-Type Oligo-p-phenylene-Containing Copolymers with High Open-Circuit Voltages and Ambient Photovoltaic Activity. J. Am. Chem. Soc. 2010, 132, 5394–5404. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Wu, J.-S.; Shih, P.-I.; Chang, C.-Y.; Jwo, P.-C.; Kao, W.-S.; Hsu, C.-S. Carbazole-Based Ladder-Type Heptacylic Arene with Aliphatic Side Chains Leading to Enhanced Efficiency of Organic Photovoltaics. Chem. Mater. 2011, 23, 2361–2369. [Google Scholar] [CrossRef]
- Sonar, P.; Williams, E.L.; Singh, S.P.; Dodabalapur, A. Thiophene-Benzothiadiazole-Thiophene (D-A-D) Based Polymers: Effect of Donor/acceptor Moieties Adjacent to D-A-D Segment on Photophysical and Photovoltaic Properties. J. Mater. Chem. 2011, 21, 10532–10541. [Google Scholar] [CrossRef]
- Hou, J.; Chen, H.; Zhang, S.; Li, G.; Yang, Y. Synthesis, Characterization, and Photovoltaic Properties of a Low Band Gap Polymer Based on Silole-Containing Polythiophenes and 2,1,3-Benzothiadiazole. J. Am. Chem. Soc. 2008, 130, 16144–16145. [Google Scholar] [CrossRef]
- Zhang, X.; Steckler, T.T.; Dasari, R.R.; Ohira, S.; Potscavage, W.J.; Tiwari, S.P.; Coppee, S.; Ellinger, S.; Barlow, S.; Bredas, J.-L.; et al. Dithienopyrrole-based Donor-Acceptor Copolymers: Low Band-gap Materials for Charge Transport, Photovoltaics and Electrochromism. J. Mater. Chem. 2010, 20, 123–134. [Google Scholar] [CrossRef]
- Yue, W.; Zhao, Y.; Shao, S.; Tian, H.; Xie, Z.; Geng, Y.; Wang, F. Novel NIR-Absorbing Conjugated Polymers for Efficient Polymer Solar Dells: Effect of Alkyl Chain Length on Device Performance. J. Mater. Chem. 2009, 19, 2199–2206. [Google Scholar] [CrossRef]
- Rout, Y.; Jang, Y.; Gobeze, H.B.; Misra, R.; D’Souza, F. Conversion of Large-Bandgap Triphenylamine−Benzothiadiazole to Low-Bandgap, Wide-Band Capturing Donor-Acceptor Systems by Tetracyanobutadiene and/or Dicyanoquinodimethane Insertion for Ultrafast Charge Separation. J. Phys. Chem. C 2019, 123, 23382–23389. [Google Scholar] [CrossRef]
- Zani, L.; Dessì, A.; Franchi, D.; Calamante, M.; Reginato, G.; Mordini, A. Transition metal-catalyzed cross-coupling methodologies for the engineering of small molecules with applications in organic electronics and photovoltaics. Coord. Chem. Rev. 2019, 392, 177–236. [Google Scholar] [CrossRef]
- Pazini, A.; Maqueira, L.; Santos, F.D.; Barreto, A.R.J.; Carvalho, R.D.; Valente, F.M.; Back, D.; Aucélio, R.Q.; Cremona, M.; Rodembusch, F.S.; et al. Designing highly luminescent aryloxy-benzothiadiazole derivatives with aggregation-induced enhanced emission. Dyes Pigments 2020, 178, 108377. [Google Scholar] [CrossRef]
- Li, F.-Z.; Li, X.-Y.; Ni, W.; Wu, Z.-W.; Lin, C.; Gurzadyan, G.G.; Kuang, J.C. Aggregation, energy transfer and intramolecular charge transfer in donor-acceptor-donor BODIPY derivative lead to single component white light emission. Dyes Pigments 2021, 195, 109736. [Google Scholar] [CrossRef]
- Yun, D.H.; Yoo, H.S.; Heo, S.W.; Song, H.J.; Moon, D.K.; Woo, J.W.; Park, Y.S. Synthesis and Photovoltaic Characterization of D/A Structure Compound Based on N-Substituted Phenothiazine and Benzothiadiazole. J. Ind. Eng. Chem. 2013, 19, 421–426. [Google Scholar] [CrossRef]
- Hinzmann, C.; Magen, O.; Hofstetter, Y.J.; Hopkinson, P.E.; Tessler, N.; Vaynzof, Y. Effect of Injection Layer Sub-Bandgap States on Electron Injection in Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 6220–6227. [Google Scholar] [CrossRef]
- Ni, F.; Wu, Z.; Zhu, Z.; Chen, T.; Wu, K.; Zhong, C.; An, K.; Wei, D.; Ma, D.; Yang, C. Teaching an Old Acceptor New Tricks: Rationally Employing 2,1,3-Benzothiadiazole as Input to Design a Highly Efficient Red Thermally Activated Delayed Fluorescence Emitter. J. Mater. Chem. C 2017, 5, 1363–1368. [Google Scholar] [CrossRef]
- Westrup, J.L.; Oenning, L.W.; Da Silva Paula, M.M.; Da Costa Duarte, R.; Rodembusch, F.S.; Frizon, T.E.A.; Da Silva, L.; Dal-Bó, A.G. New Photoactive D-π-A-π-D Benzothiadiazole Derivative: Synthesis, Thermal and Photophysical Properties. Dyes Pigments 2016, 126, 209–217. [Google Scholar] [CrossRef]
- Anant, P.; Mangold, H.; Lucas, N.T.; Laquai, F.; Jacob, J. Synthesis and Characterization of Donor–Acceptor Type 4,4′-Bis(2,1,3-Benzothiadiazole)-Based Copolymers. Polymer 2011, 52, 4442–4450. [Google Scholar] [CrossRef]
- Ke, M.; Tan, X.; Wang, Y.; Li, B.; Zeng, X.; Miao, X.; Cheng, X.; Deng, W. Solvent-Dependent Molecular Isomerization and 2D Self-Assembled Phase Transitions of Benzothiadiazole-Based π-Conjugated Fluorophore. J. Phys. Chem. C 2021, 125, 19325–19332. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.C.; Wong, W.Y. Recent progress of electronic materials based on 2,1,3-benzothiadiazole and its derivatives: Synthesis and their application in organic light-emitting diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Correa, J.R.; Spencer, J. Fluorescent Benzothiadiazole Derivatives as Fluorescence Imaging Dyes: A Decade of New Generation Probes. Chem. Eur. J. 2021, 27, 1–29. [Google Scholar] [CrossRef]
- Ishii, T.; Ikeda, K.; Ogawa, M.; Kusakaki, Y. Light-emitting properties of donor-acceptor and donor-acceptor-donor dyes in solution, solid, and aggregated states: Structure–property relationship of emission behavior. RSC Adv. 2015, 5, 89171. [Google Scholar] [CrossRef]
- Cui, L.-S.; Nomura, H.; Geng, Y.; Kim, J.U.; Nakanotani, H.; Adachi, C. Controlling Singlet-Triplet Energy Splitting for Deep-Blue Thermally Activated Delayed Fluorescence Emitters. Angew. Chem. Int. Ed. 2017, 56, 1571. [Google Scholar] [CrossRef]
- Pathak, A.; Thomas, K.R.J.; Singh, M.; Jou, J.-H. Fine-Tuning of Photophysical and Electroluminescence Properties of Benzothiadiazole-Based Emitters by Methyl Substitution. J. Org. Chem. 2017, 82, 11512–11523. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Lypenko, D.A.; Dmitriev, A.V.; Pozin, S.I.; Topchiy, M.A.; Asachenko, A.F.; Loginov, D.A.; Osipov, S.N. Synthesis and optical properties of novel unsymmetrically substituted benzothiadiazole-based luminophores. Mendeleev Commun. 2021, 31, 33–35. [Google Scholar] [CrossRef]
- Pazini, A.; Maqueira, L.; Stieler, R.; Aucélio, R.Q.; Limberger, J. Synthesis, characterization and photophysical properties of luminescent non-symmetric 4-pyridyl benzothiadiazole derivatives. J. Mol. Struct. 2017, 1131, 181–189. [Google Scholar] [CrossRef]
- Mancilha, F.S.; Barloy, L.; Rodembusch, F.S.; Dupont, J.; Pfeffer, M. Cyclopalladated complexes of 4-aryl-2,1,3-benzothiadiazoles: New emitters in solution at room temperature. Dalton Trans. 2011, 40, 10535–10544. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Z.; Huang, Z.; Liu, S.; Liu, P.; Wang, Y. Expression of anti-Kasha’s emission from amino benzothiadiazole and its utilization for fluorescent chemosensors and organic light emitting materials. J. Mater. Chem. C 2018, 6, 7864–7873. [Google Scholar] [CrossRef]
- Davalos, A.R.; Sylvester, E.; Diver, S.T. Macrocyclic N-Heterocyclic Carbenes: Synthesis and Catalytic Applications. Organometallics 2019, 38, 2338–2346. [Google Scholar] [CrossRef]
- Gordon, R.D.; Yang, R.F. The near ultraviolet spectra of 2,1,3-benzothiadiazole and its deuterated and substituted derivatives. J. Mol. Spectrosc. 1971, 39, 295–320. [Google Scholar] [CrossRef]
- Parker, V.C.A. Photoluminescence of Solutions; Elsevier Publishing Co.: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1968. [Google Scholar]
- Chen, X.; Ma, D.; Liu, T.; Chen, Z.; Yang, Z.; Zhao, J.; Yang, X.; Zhang, Y.; Chi, Z. Hybridized Local and Charge-Transfer Excited-State Fluorophores through the Regulation of the Donor–Acceptor Torsional Angle for Highly Efficient Organic Light-Emitting Diodes. CCS Chem. 2021, 3, 1285–1295. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA; London, UK, 1986. [Google Scholar]
- Dias, F.B.; Santos, J.; Graves, D.R.; Data, P.; Nobuyasu, R.S.; Fox, M.A.; Batsanov, A.S.; Palmeira, T.; Berberan-Santos, M.N.; Bryce, M.R.; et al. The Role of Local Triplet Excited States and D-A Relative Orientation in Thermally Activated Delayed Fluorescence: Photophysics and Devices. Adv. Sci. 2016, 3, 1600080. [Google Scholar] [CrossRef] [Green Version]
- Hosokai, T.; Matsuzaki, H.; Nakanotani, H.; Tokumaru, K.; Tsutsui, T.; Furube, A.; Nasu, K.; Nomura, H.; Yahiro, M.; Adachi, C. Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states. Sci. Adv. 2017, 3, e1603282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.A.; Hatchard, C.G. Triplet-singlet emission in fluid solutions. Phosphorescence of eosin. Trans. Faraday Soc. 1961, 57, 1894–1904. [Google Scholar] [CrossRef]
- Wang, H.; Xie, L.; Peng, Q.; Meng, L.; Wang, Y.; Yi, Y.; Wang, P. Novel Thermally Activated Delayed Fluorescence Materials–Thioxanthone Derivatives and Their Applications for Highly Efficient OLEDs. Adv. Mater. 2014, 26, 5198–5204. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Huang, W.; Einzinger, M.; Maurano, A.; Zhu, T.; Tiepelt, J.; Yu, C.; Chae, H.S.; Van Voorhis, T.; Baldo, M.A.; Buchwald, S.L. Large Increase in External Quantum Efficiency by Dihedral Angle Tuning in a Sky-Blue Thermally Activated Delayed Fluorescence Emitter. Adv. Opt. Mater. 2019, 7, 1900476. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.Y. Engineering of Mixed Host for High External Quantum Efficiency above 25% in Green Thermally Activated Delayed Fluorescence Device. Adv. Funct. Mater. 2014, 24, 3970–3977. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Liu, S.-W.; Chen, C.-T.; Lee, J.-H. Emitting layer thickness dependence of color stability in phosphorescent organic light-emitting devices. Org. Electron. 2010, 11, 1500–1506. [Google Scholar] [CrossRef]
- Lypenko, D.A.; Nosova, G.I.; Berezin, I.A.; Tameev, A.R.; Mal’tsev, E.I. Role of benzothiadiazole substituents in white electroluminescent single macromolecules of fluorene-based copolymers. Mendeleev Commun. 2020, 30, 165–167. [Google Scholar] [CrossRef]
- Lee, J.; Jang, M.; Lee, S.M.; Yoo, D.; Shin, T.J.; Oh, J.H.; Yang, C. Fluorinated Benzothiadiazole (BT) Groups as a Powerful Unit for High-Performance Electron-Transporting Polymers. ACS Appl. Mater. Interfaces 2014, 6, 20390–20399. [Google Scholar] [CrossRef]
- Neto, A.D.B.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Angioni, E.; Chapran, M.; Ivaniuk, K.; Kostiv, N.; Cherpak, V.; Stakhira, P.; Lazauskas, A.; Tamulevičius, S.; Volyniuk, D.; Findlay, N.J.; et al. A single emitting layer white OLED based on exciplex interface emission. J. Mater. Chem. C 2016, 4, 3851–3856. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Pivrikas, A.; Xu, B.; Liu, Y.; Xu, W.; van Loosdrecht, P.H.M.; Tian, W. Measuring electron and hole mobilities in organic systems: Chargeselective CELIV. Synth. Met. 2015, 203, 187–191. [Google Scholar] [CrossRef]
- Kim, S.-K.; Yang, B.; Ma, Y.; Lee, J.-H.; Park, J.-W. Exceedingly efficient deep-blue electroluminescence from new anthracenes obtained using rational molecular design. J. Mater. Chem. 2008, 18, 3376–3384. [Google Scholar] [CrossRef]
- Kuo, H.-H.; Chen, Y.-T.; Devereux, L.R.; Wu, C.-C.; Fox, M.A.; Kuei, C.-Y.; Chi, Y.; Lee, G.-H. Bis-Tridentate Ir(III) Metal Phosphors for Efficient Deep-Blue Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702464. [Google Scholar] [CrossRef] [Green Version]
- Ye, T.; Chen, J.; Ma, D. Electroluminescence of poly(N-vinylcarbazole) films: Fluorescence, phosphorescence and electromers. Phys. Chem. Chem. Phys. 2010, 12, 15410–15413. [Google Scholar] [CrossRef]
- Tokarev, S.D.; Sotnikova, Y.A.; Anisimov, A.V.; Fedorov, Y.V.; Jonusauskas, G.; Lypenko, D.A.; Malov, V.V.; Tameev, A.R.; Mal’tsevd, E.I.; Fedorova, O.A. Donor–acceptor (E)-2-[2-(2,2′-bithiophen-5-yl)vinyl]benzo[d]thiazole: Synthesis, optical, electrochemical studies and charge transport characteristics. Mendeleev Commun. 2019, 29, 567–569. [Google Scholar] [CrossRef]
- Malov, V.V.; Ghosh, T.; Nair, V.C.; Maslov, M.M.; Katin, K.P.; Narayanan Unni, K.N.; Tameev, A.R. Hole mobility in thieno[3,2-b]thiophene oligomers. Mendeleev Commun. 2019, 29, 218–219. [Google Scholar] [CrossRef]
- Tokarev, S.D.; Fedorov, Y.V.; Moiseeva, A.A.; Jonusauskas, G.; Lypenko, D.A.; Aleksandrov, A.E.; Tameev, A.R.; Maltsev, E.I.; Nosova, G.I.; Elena, V.; et al. Modification of the carrier mobility of conducting PF-EP polymer by formation of their composites with thiophene derivatives. Org. Electron. 2020, 78, 105586. [Google Scholar] [CrossRef]
- Gelfand, N.; Freidzon, A.; Vovna, V. Theoretical insights into UV-Vis absorption spectra of difluoroboron b-diketonates with an extended p system: An analysis based on DFT and TD-DFT calculations. Spectrochim. Acta Part A 2019, 216, 161–172. [Google Scholar] [CrossRef]
- Sandberg, O.J.; Nyman, M.; Dahlstrom, S.; Sanden, S.; Torngren, B.; Smatt, J.-H.; Osterbacka, R. On the validity of MIS-CELIV for mobility determination in organic thin-film devices. Appl. Phys. Lett. 2017, 110, 153504. [Google Scholar] [CrossRef]









| Substance | λabsmax 298 K, nm b | λPLmax 298 K, nm b | λPLmax 77 K, nm b | λPmax 77 K, nm b | λPLmax 298 K, nm c | φPL 298 K, % c |
|---|---|---|---|---|---|---|
| D1 | 420 | 536 | 508 | 520 | 539 | 98.6 |
| D2 | 404 | 506 | 482 | 488 | 523 | 91.1 |
| D3 | 412 | 517 | 498 | 502 | 534 | 49.2 |
| Material | LUMO | Electron Mobility | HOMO | Hole Mobility |
|---|---|---|---|---|
| mCP | −2.400 | 3.37·10−4 | −5.900 | 3.89·10−4 |
| D1 | −3.452 | 3.86·10−4 | −5.238 | 2.49·10−4 |
| D2 | −3.442 | 1.88·10−3 | −5.968 | 8.73·10−4 |
| D3 | −3.263 | 2.68·10−3 | −5.785 | 3.97·10−3 |
| Ligt-Emitting Layer | Uon, V | Brightness cd/m2 U = 15V | Max. Efficiency | CIE | λmax EL, nm | ||
|---|---|---|---|---|---|---|---|
| Current cd/A | Luminous lm/W | X | y | ||||
| D1/mCP | 4.4 | 9300 | 2.04 | 1.09 | 0.354 | 0.580 | 542 |
| D2/mCP | 4.6 | 2270 | 1.80 | 0.75 | 0.330 | 0.578 | 539 |
| D3/mCP | 4.0 | 10,500 | 2.70 | 0.91 | 0.380 | 0.577 | 558 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribanov, P.S.; Loginov, D.A.; Lypenko, D.A.; Dmitriev, A.V.; Pozin, S.I.; Aleksandrov, A.E.; Tameev, A.R.; Martynov, I.L.; Chernyadyev, A.Y.; Osipov, S.N. New Unsymmetrically Substituted Benzothiadiazole-Based Luminophores: Synthesis, Optical, Electrochemical Studies, Charge Transport, and Electroluminescent Characteristics. Molecules 2021, 26, 7596. https://doi.org/10.3390/molecules26247596
Gribanov PS, Loginov DA, Lypenko DA, Dmitriev AV, Pozin SI, Aleksandrov AE, Tameev AR, Martynov IL, Chernyadyev AY, Osipov SN. New Unsymmetrically Substituted Benzothiadiazole-Based Luminophores: Synthesis, Optical, Electrochemical Studies, Charge Transport, and Electroluminescent Characteristics. Molecules. 2021; 26(24):7596. https://doi.org/10.3390/molecules26247596
Chicago/Turabian StyleGribanov, Pavel S., Dmitry A. Loginov, Dmitry A. Lypenko, Artem V. Dmitriev, Sergey I. Pozin, Alexey E. Aleksandrov, Alexey R. Tameev, Igor L. Martynov, Andrey Yu. Chernyadyev, and Sergey N. Osipov. 2021. "New Unsymmetrically Substituted Benzothiadiazole-Based Luminophores: Synthesis, Optical, Electrochemical Studies, Charge Transport, and Electroluminescent Characteristics" Molecules 26, no. 24: 7596. https://doi.org/10.3390/molecules26247596