The Antioxidant Phytochemical Schisandrin A Promotes Neural Cell Proliferation and Differentiation after Ischemic Brain Injury
Abstract
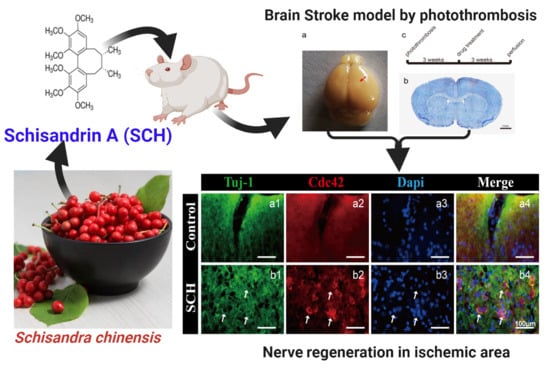
:1. Introduction
2. Results and Discussion
2.1. Neural Progenitor Cell (NPC) Regeneration after Cerebral Ischemia Caused by Photothrombosis
2.2. SCH Promotes In Vitro Proliferation of NPCs
2.3. Effect of SCH on Differentiation
2.4. Cdc42 Expression Level Analysis
3. Materials and Methods
3.1. Chemicals and Animals
3.2. Induction of Ischemic Stroke and Preparation of Tissues for Photothrombosis Model Detection
3.2.1. Immunofluorescence Assay
3.2.2. Cell Line Culture of NPCs and Primary Cortical Neuron Culture
3.2.3. Treatment with SCH
3.2.4. NPCs Cell Curve and Detection of Cell Cycle
3.2.5. NPCs Differentiation Neurons in Co-Culture System
3.2.6. Western Blot Assay
3.3. Statistical Analysis
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pandian, J.D.; Kalkonde, Y.; Sebastian, I.A.; Felix, C.; Urimubenshi, G.; Bosch, J. Stroke systems of care in low-income and middle-income countries: Challenges and opportunities. Lancet 2020, 396, 1443–1451. [Google Scholar] [CrossRef]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2021, 65, 101211. [Google Scholar] [CrossRef]
- Jodeiri Farshbaf, M.; Alvina, K. Multiple roles in neuroprotection for the exercise derived myokine irisin. Front. Aging Neurosci. 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ahn, S.M.; Pak, M.E.; Jung, D.H.; Lee, S.Y.; Shin, H.K.; Choi, B.T. Positive effects of alpha-asarone on transplanted neural progenitor cells in a murine model of ischemic stroke. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 51, 151–161. [Google Scholar]
- Liu, L.; Zhao, X.; Silva, M.; Li, S.; Xing, X.; Zheng, W. Artemisinin protects motoneurons against axotomy-induced apoptosis through activation of the pka-akt signaling pathway and promotes neural stem/progenitor cells differentiation into neun(+) neurons. Pharmacol. Res. 2020, 159, 105049. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Llorens-Bobadilla, E.; Magnusson, J.P.; Frisén, J. A widespread neurogenic potential of neocortical astrocytes is induced by injury. Cell Stem Cell 2020, 27, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qi, Y.; Zhao, T.; Fang, J.; Liu, X.; Xu, T.; Yang, Q.; Dai, X. Ngf increases fgf2 expression and promotes endothelial cell migration and tube formation through pi3k/akt and erk/mapk pathways in human chondrocytes. Osteoarthr. Cartil. 2019, 27, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, L.; Gouda, M.; Buttar, H.S. Pomegranate, its components and modern deliverable formulations as potential botanicals in the prevention and treatment of various cancers. Curr. Drug Deliv. 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Gouda, M.; Huang, Z.; Liu, Y.; He, Y.; Li, X. Physicochemical impact of bioactive terpenes on the microalgae biomass structural characteristics. Bioresour. Technol. 2021, 334, 125232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Gouda, M.M.; Hussein, L.A.; Ahmed, N.C.; Vos, P.W.; Mohammad, M.A. Role of melt curve analysis in interpretation of nutrigenomics’ microrna expression data. Cancer Genom. Proteom. 2017, 14, 469–481. [Google Scholar]
- Gouda, M.; El-Din Bekhit, A.; Tang, Y.; Huang, Y.; Huang, L.; He, Y.; Li, X. Recent innovations of ultrasound green technology in herbal phytochemistry: A review. Ultrason. Sonochemistry 2021, 73, 105538. [Google Scholar] [CrossRef]
- Lv, J.M.; Gouda, M.; Zhu, Y.Y.; Ye, X.Q.; Chen, J.C. Ultrasound-assisted extraction optimization of proanthocyanidins from kiwi (actinidia chinensis) leaves and evaluation of its antioxidant activity. Antioxidants 2021, 10, 1317. [Google Scholar] [CrossRef]
- Cai, N.N.; Wang, Z.Z.; Zhu, X.C.; Jiang, Y.; Zhu, W.Q.; Yang, R.; Zhang, X.M. Schisandrin a and b enhance the dentate gyrus neurogenesis in mouse hippocampus. J. Chem. Neuroanat. 2020, 105, 101751. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cong, L.; Wang, C.; Li, H.; Zhang, C.; Guan, X.; Liu, P.; Xie, Y.; Chen, J.; Sun, J. Pharmacokinetics and distribution of schisandrol a and its major metabolites in rats. Xenobiotica 2019, 49, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of schisandra chinensis (turcz.) baill. (chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Guo, J.L.; Wang, L.; Zhong, X.; Yao, W.F.; Gao, H.; Liu, M.Y. Natural products as pharmacological modulators of mitochondrial dysfunctions for the treatments of alzheimer’s disease: A comprehensive review. Eur. J. Med. Chem. 2021, 218, 113401. [Google Scholar] [CrossRef]
- Zhi, Y.; Jin, Y.; Pan, L.; Zhang, A.; Liu, F. Schisandrin a ameliorates mptp-induced parkinson’s disease in a mouse model via regulation of brain autophagy. Arch. Pharmacal Res. 2019, 42, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, Y.; Zhang, L.; Gong, X.; Shi, W.; Liu, L.; Wang, X. Therapeutic effect of schisandrin a on avian colibacillosis through gut-liver axis. Poult. Sci. 2021, 100, 101371. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Turner, P.C.; Co, V.A.; Wang, M.F.; Amiri, K.M.A.; El-Nezami, H. Schisandrin a protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 2019, 9, 19173. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, X.; Jing, H.; Yan, T.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Combination of schisandrin and nootkatone exerts neuroprotective effect in alzheimer’s disease mice model. Metab. Brain Dis. 2019, 34, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, L.; Xia, Y.; Chen, J.; Hua, M.; Sun, Y. Schisandrin b attenuates hepatic stellate cell activation and promotes apoptosis to protect against liver fibrosis. Molecules 2021, 26, 6882. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, L.; Price, M.; Bezzi, P.; Hirt, L. Spatio-temporal overview of neuroinflammation in an experimental mouse stroke model. Sci. Rep. 2019, 9, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindvall, O.; Kokaia, Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a019034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.H.; Bouchard-Cannon, P.; Hegazi, S.; Lowden, C.; Fung, S.W.; Chiang, C.K.; Ness, R.W.; Cheng, H.M. Sox2-dependent transcription in clock neurons promotes the robustness of the central circadian pacemaker. Cell Rep. 2019, 26, 3191–3202. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Chung, S.H.; Jiang, P.; Dehghan, S.; Deng, W. Development of glial restricted human neural stem cells for oligodendrocyte differentiation in vitro and in vivo. Sci. Rep. 2019, 9, 9013. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Abbasi, F.; Koch, W.T.; Mostowski, H.; Varadkar, P.; McCright, B. Evaluation of the differentiation status of neural stem cells based on cell morphology and the expression of notch and sox2. Cytotherapy 2018, 20, 1472–1485. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, A.; Garcia-Concejo, A.; Leon-Lobera, F.; Rodriguez, R.E. Morphine delays neural stem cells differentiation by facilitating nestin overexpression. Biochim. et Biophys. Acta. Gen. Subj. 2018, 1862, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Spijkers, X.M.; Pasteuning-Vuhman, S.; Dorleijn, J.C.; Vulto, P.; Wevers, N.R.; Pasterkamp, R.J. A directional 3d neurite outgrowth model for studying motor axon biology and disease. Sci. Rep. 2021, 11, 2080. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, J.C.; Yao, C.Y.; Wu, Y.; Abdelgawad, A.F.; Yao, S.L.; Yuan, S.Y. Critical role of astrocytic interleukin-17 a in post-stroke survival and neuronal differentiation of neural precursor cells in adult mice. Cell Death Dis. 2016, 7, e2273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, F.; Zhao, W.; Li, J.; Li, Q.; Wang, W. Protective effects of allicin against ischemic stroke in a rat model of middle cerebral artery occlusion. Mol. Med. Rep. 2015, 12, 3734–3738. [Google Scholar] [CrossRef] [PubMed]
- Dranovsky, A.; Picchini, A.M.; Moadel, T.; Sisti, A.C.; Yamada, A.; Kimura, S.; Leonardo, E.D.; Hen, R. Experience dictates stem cell fate in the adult hippocampus. Neuron 2011, 70, 908–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [Green Version]
- Govek, E.E.; Wu, Z.; Acehan, D.; Molina, H.; Rivera, K.; Zhu, X.; Fang, Y.; Tessier-Lavigne, M.; Hatten, M.E. Cdc42 regulates neuronal polarity during cerebellar axon formation and glial-guided migration. iScience 2018, 1, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Quadri, R.; Galli, M.; Galati, E.; Rotondo, G.; Gallo, G.R.; Panigada, D.; Plevani, P.; Muzi-Falconi, M. Haspin regulates ras localization to promote cdc24-driven mitotic depolarization. Cell Discov. 2020, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Xu, C.; Funahashi, Y.; Namba, T.; Kaibuchi, K. Neuronal polarization. Development 2015, 142, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-P.; He, H.-L.; Hu, S.-L.; Han, J.-B.; Huang, L.-L.; Xu, J.-Y.; Xie, J.-F.; Liu, A.-R.; Yang, Y.; Qiu, H.-B. Ang ii-at2r increases mesenchymal stem cell migration by signaling through the fak and rhoa/cdc42 pathways in vitro. Stem Cell Res. Ther. 2017, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Rappaz, B.; Lai Wing Sun, K.; Correia, J.P.; Wiseman, P.W.; Kennedy, T.E. Flim fret visualization of cdc42 activation by netrin-1 in embryonic spinal commissural neuron growth cones. PLoS ONE 2016, 11, e0159405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkov, M.; Gulyaev, M.; Kiseleva, E.; Andreev-Andrievskiy, A.; Gorbacheva, L. Methods for detection of brain injury after photothrombosis-induced ischemia in mice: Characteristics and new aspects of their application. J. Neurosci. Methods 2020, 329, 108457. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, W.; Chen, J.; Zhang, X.; Shi, L.; Ren, C. Remote limb preconditioning protects against ischemia-induced neuronal death through ameliorating neuronal oxidative DNA damage and parthanatos. J. Neurol. Sci. 2016, 366, 8–17. [Google Scholar] [CrossRef]
- Taccola, C.; Barneoud, P.; Cartot-Cotton, S.; Valente, D.; Schussler, N.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Mignon, V.; Curis, E.; et al. Modifications of physical and functional integrity of the blood-brain barrier in an inducible mouse model of neurodegeneration. Neuropharmacology 2021, 191, 108588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, B.; Ai, Z.; Xiang, Z.; Zhang, K.; Qiu, X.; Chen, Y.; Li, Y.; Rizak, J.D.; Niu, Y.; et al. A robust single primate neuroepithelial cell clonal expansion system for neural tube development and disease studies. Stem Cell Rep. 2016, 6, 228–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumamaru, H.; Kadoya, K.; Adler, A.F.; Takashima, Y.; Graham, L.; Coppola, G.; Tuszynski, M.H. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 2018, 15, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Min Lai, S.; Keighley, J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology 2000, 39, 835–841. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Cong, Y.-L.; Liu, Y.; Jin, B.; Si, L.; Wang, A.-B.; Cai, H.; Che, G.-Y.; Tang, B.; Wang, C.-F.; et al. Schisandrin a and b affect subventricular zone neurogenesis in mouse. Eur. J. Pharmacol. 2014, 740, 552–559. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, W.; Gouda, M.; Cai, E.; Wang, R.; Xu, W.; Wu, Y.; Munekata, P.E.S.; Lorenzo, J.M. The Antioxidant Phytochemical Schisandrin A Promotes Neural Cell Proliferation and Differentiation after Ischemic Brain Injury. Molecules 2021, 26, 7466. https://doi.org/10.3390/molecules26247466
Zong W, Gouda M, Cai E, Wang R, Xu W, Wu Y, Munekata PES, Lorenzo JM. The Antioxidant Phytochemical Schisandrin A Promotes Neural Cell Proliferation and Differentiation after Ischemic Brain Injury. Molecules. 2021; 26(24):7466. https://doi.org/10.3390/molecules26247466
Chicago/Turabian StyleZong, Wentian, Mostafa Gouda, Enli Cai, Ruofeng Wang, Weijie Xu, Yuming Wu, Paulo E. S. Munekata, and José M. Lorenzo. 2021. "The Antioxidant Phytochemical Schisandrin A Promotes Neural Cell Proliferation and Differentiation after Ischemic Brain Injury" Molecules 26, no. 24: 7466. https://doi.org/10.3390/molecules26247466