From a Well-Defined Organozinc Precursor to Diverse Luminescent Coordination Polymers Based on Zn(II)-Quinolinate Building Units Interconnected by Mixed Ligand Systems
Abstract
:1. Introduction
2. Results and Discussion
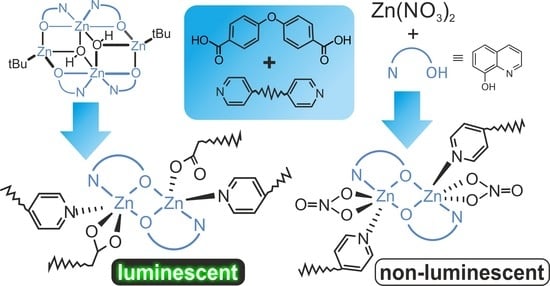
2.1. Synthesis of Molecular Building Units and Coordination Polymers Based on Zn(II)-Quinolinate Species
2.2. Single Crystal X-ray Diffraction and Structure Analysis
2.3. FTIR Spectroscopic Study
2.4. Photophysical Characterization
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis of [(Zn4(q)4(obc)2(DEF)2] (CP1)
4.3. Synthesis of [(Znq)2(bpe)(obc)] (CP2)
4.4. Synthesis of [(Znq)2(bpene)(obc)] (CP3)
4.5. Synthesis of the Molecular Cluster [Zn4(q)6(bipy)2(obc)2] (HP4)
4.6. Synthesis of [Zn2(q)2(NO3)2(bipy)] (CP5)
4.7. Single-Crystal X-ray Diffraction Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Jiang, H.; Sun, L. Metal−Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Edem, B.; Huang, J.; Zeng, J.; Subhan, F.; Feng, F.; Zhang, Y.; Qiu, Z.; Aslam, S.; Li, G.; Yan, Z. Magnetic Metal–Organic Framework Composites for Environmental Monitoring and Remediation. Coord. Chem. Rev. 2020, 413, 213261. [Google Scholar] [CrossRef]
- Connolly, B.M.; Mehta, J.P.; Moghadam, P.Z.; Wheatley, A.E.H.; Fairen-Jimenez, D. From Synthesis to Applications: Metal–Organic Frameworks for an Environmentally Sustainable Future. Curr. Opin. Green Sustain. Chem. 2018, 12, 47–56. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, H.; Wang, Y.; Ying, Y. Recent Advances in the Rational Synthesis and Sensing Applications of Metal-Organic Framework Biocomposites. Coord. Chem. Rev. 2019, 387, 60–78. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Li, S.J.; Ghosh, S.K.; Lustig, W.P.; Desai, A. V Metal-Organic Frameworks: Functional Luminescent and Photonic Materials for Sensing Applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Pan, Y.; Kumar, A.; Trivedi, M.; Kumar, A. Recent Developments in Luminescent Coordination Polymers: Designing Strategies, Sensing Application and Theoretical Evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Zhao, H.; Zou, Q.; Sun, S.; Yu, C.; Zhang, X.; Li, R.; Fu, Y. Theranostic Metal-Organic Framework Core-Shell Composites for Magnetic Resonance Imaging and Drug Delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-S. Metal–Organic Frameworks for Biosensing and Bioimaging Applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Li, X.; Cui, Y. Cation-Dependent Nonlinear Optical Behavior in an Octupolar 3D Anionic Metal-Organic Framework. Angew. Chem. Int. Ed. 2007, 46, 6301–6304. [Google Scholar] [CrossRef]
- Huang, X.; Li, Q.; Xiao, X.; Jia, S.; Li, Y.; Duan, Z.; Bai, L.; Yuan, Z. Nonlinear-Optical Behaviors of a Chiral Metal−Organic Framework Comprised of an Unusual Multioriented Double-Helix Structure. Inorg. Chem. 2018, 57, 6210–6213. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-Based Electronic and Optoelectronic Devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, S.; Chen, M.; Wasielewski, M.R.; Farha, O.K.; Hupp, J.T. Boosting Transport Distances for Molecular Excitons within Photoexcited Metal−Organic Framework Films. ACS Appl. Nano Mater. 2018, 10, 34409–34417. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Li, J. Luminescent Metal–Organic Frameworks and Coordination Polymers as Alternative Phosphors for Energy Efficient Lighting Devices. Coord. Chem. Rev. 2018, 373, 116–147. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Wang, J.; Du, C.; Deng, Z. Tunable Photoluminescence and Direct White-Light Emission in Mg-Based Coordination Networks. Chem. Commun. 2015, 51, 157–160. [Google Scholar] [CrossRef]
- Jensen, S.; Tan, K.; Lustig, W.P.; Kilin, D.S.; Li, J.; Chabal, Y.J.; Thonhauser, T. Structure-Driven Photoluminescence Enhancement in a Zn-Based Metal−Organic Framework. Chem. Mater. 2019, 31, 7933–7940. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Qin, J.; Pang, J.; Zhang, P.; Zhang, Y.; Huang, Y.; Drake, H.F.; Liu, W.R.; Zhou, H. Stepwise Assembly of Turn-on Fluorescence Sensors in Multicomponent Metal–Organic Frameworks for In Vitro Cyanide. Angew. Chem. Int. Ed. 2020, 59, 9319–9323. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Muller-Buschbaum, K. Engineering Metal-Based Luminescence in Coordination Polymers and Metal–Organic Frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Samanta, P.; Desai, A.V.; Ghosh, S.K. Guest-Responsive Metal−Organic Frameworks as Scaffolds for Separation and Sensing Applications. Acc. Chem. Res. 2017, 50, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.P. The Reaction of 8-Quinolinol. Chem. Rev. 1956, 56, 271–297. [Google Scholar] [CrossRef]
- Albrecht, M.; Fiege, M.; Osetska, O. 8-Hydroxyquinolines in Metallosupramolecular Chemistry. Coord. Chem. Rev. 2008, 252, 812–824. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A Review of Their Metal Chelating Properties and Medicinal Applications. Drug Des. Devel. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.W.; Vanslyke, S.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A.; et al. Purely Organic Electroluminescent Material Realizing 100% Conversion from Electricity to Light. Nat. Commun. 2015, 6, 8476–8483. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Yang, K.; Ma, W.; Zhang, L.; Yuan, G. A Highly STable 8-Hydroxyquinolinate-Based Metal–Organic Framework as a Selective Fluorescence Sensor for Fe3+, Cr2O72− and Nitroaromatic Explosives. Inorg. Chem. Front. 2020, 7, 4387–4395. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Li, X.; Tian, Y.; Yuan, G. Five 8-Hydroxyquinolinate-Based Coordination Polymers with Tunable Structures and Photoluminescent Properties for Sensing Nitroaromatics. Dalton Trans. 2015, 44, 401–410. [Google Scholar] [CrossRef]
- Zhang, L.; Rong, L.; Hu, G.; Jin, S.; Jia, W.; Liu, J.; Yuan, G. Six Zn (II) and Cd (II) Coordination Polymers Assembled from a Similar Binuclear Building Unit: Tunable Structures and Luminescence Properties. Dalton Trans. 2015, 44, 6731–6739. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Zhang, L.; Liu, S.; Yuan, G. 8-Hydroxyquinolinate-Based Metal-Organic Frameworks: Synthesis, Tunable Luminescent Properties, and Highly Sensitive Detection of Small Molecules and Metal Ions. Inorg. Chem. 2018, 58, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Huo, Y.; Nie, X.; Jiang, H.; Liu, B.; Fang, X.; Zhao, F. Controllable Supramolecular Structures and Luminescent Properties of Unique Trimeric Zn(II) 8-Hydroxyquinolinates Tuned by Functional Substituents. Dalton Trans. 2013, 42, 2921–2929. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zhang, Q.; Wang, Z.; Song, K.; Yuan, X.; Wang, Y.; Zhang, L. Assembly of Four 8-Quinolinate-Based Multinuclear Complexes: The Effect of Substituents on Core Structures and Photoluminescent Properties. Inorg. Chem. Front. 2017, 4, 764–772. [Google Scholar] [CrossRef]
- Yuan, G.; Shan, W.; Xuelong, Q.; Ma, L.; Huo, Y. Self-Assembly of Five 8-Hydroxyquinolinate-Based Complexes: Tunable Core, Supramolecular Structure and Photoluminescence Properties. Chem. Asian J. 2014, 9, 1913–1921. [Google Scholar] [CrossRef]
- Yuan, G.; Hu, G.; Shan, W.; Jin, S.; Gu, Q.; Chen, J. Structural and Luminescent Modulation in 8-Hydroxyquinolinate-Based Coordination Polymers by Varying Dicarboxylic Acids. Dalton Trans. 2015, 44, 17774–17783. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, C.-P.; Liu, C.-S.; Fang, S.-M. Design and Construction of Coordination Polymers with Mixed-Ligand Synthetic Strategy. Coord. Chem. Rev. 2013, 257, 1282–1305. [Google Scholar] [CrossRef]
- De Carvalho, A.B.; de Souza, Í.P.; de Andrade, L.M.; Binatti, I.; Pedroso, E.F.; Krambrock, K.; Oliveira, W.X.C.; Pereira-Maia, E.C.; Silva-Caldeira, P.P. Novel Copper (II) Coordination Polymer Containing the Drugs Nalidixic Acid and 8-Hydroxyquinoline: Evaluation of the Structural, Magnetic, Electronic and Antitumor Properties. Polyhedron 2018, 156, 312–319. [Google Scholar] [CrossRef]
- Xiang, J.; Jia, L.; Man, W.; Qian, K.; Yiu, S.; Lee, G.; Peng, S.-M.; Gao, S.; Lau, T.-C. Novel Heterobimetallic Ruthenium(III)–cobalt(II) Compounds Constructed from Trans-[RuIII(Q)2(CN)2]− (Q = 8-Quinolinolato): Synthesis, Structures and Magnetic Propertiesw. Chem. Commun. 2011, 47, 8694–8696. [Google Scholar] [CrossRef]
- Montes, V.A.; Zyryanov, G.V.; Danilov, E.; Agarwal, N.; Palacios, M.A.; Anzenbacher, P. Ultrafast Energy Transfer in Oligofluorene-Aluminum Bis(8-Hydroxyquinoline) Acetylacetone Coordination Polymers. J. Am. Chem. Soc. 2009, 15, 1787–1795. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, H.H.; Chen, Z.R.; Huang, C.C.; Huang, X.H.; Feng, M.; Lin, Y. A Series of Lead (II)/Iodine Hybrid Polymers Based on 1-D and 2-D Metal–Organic Motifs Linked by Different Organic Conjugated Ligands. Cryst. Eng. Comm. 2008, 10, 770–777. [Google Scholar] [CrossRef]
- Guochun, Z.; Chengfang, Q.; Jiehui, L.; Qing, W.; Zhengqiang, X.; Sanping, C. Hydrothermal Synthesis, Structure and Property of Transition Metal (Mn, Zn, Cd or Pb) Coordination Frameworks Using Quinoline-8-Oxy-Acetate Acid and Dicarboxylic Acid as Ligands. Chem. Res. Chin. Univ. 2015, 31, 489–497. [Google Scholar] [CrossRef]
- Sokołowski, K.; Justyniak, I.; Śliwiński, W.; Sołtys, K.; Tulewicz, A.; Kornowicz, A.; Moszyński, R.; Lipkowski, J.; Lewiński, J. Towards a New Family of Photoluminescent Organozinc 8-Hydroxyquinolinates with a High Propensity to Form Noncovalent Porous Materials. Chem. Eur. J. 2012, 18, 5637–5645. [Google Scholar] [CrossRef]
- Sokołowski, K.; Bury, W.; Justyniak, I.; Fairen-Jimenez, D.; Sołtys, K.; Prochowicz, D.; Yang, S.; Schröder, M.; Lewiński, J. Permanent Porosity Derived from the Self-Assembly of Highly Luminescent Molecular Zinc Carbonate Nanoclusters. Angew. Chem. Int. Ed. 2013, 52, 13414–13418. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, K.; Bury, W.; Justyniak, I.; Cieślak, A.M.; Wolska, M.; Sołtys, K.; Dzięcielewski, I.; Lewiński, J. Activation of CO2 by TBuZnOH Species: Efficient Routes to Novel Nanomaterials Based on Zinc Carbonates. Chem. Commun. 2013, 49, 5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszczyński, M.K.; Justyniak, I.; Zelga, K.; Lewiński, J. From Ethylzinc Guanidinate to [Zn10O4]-Supertetrahedron. Dalton Trans. 2017, 46, 12404–12407. [Google Scholar] [CrossRef] [Green Version]
- Mąkolski, Ł.; Szejko, V.; Zelga, K.; Tulewicz, A.; Bernatowicz, P.; Justyniak, I.; Lewiński, J. Unravelling Structural Mysteries of Simple Organozinc Alkoxides. Chem. Eur. J. 2021, 27, 5666–5674. [Google Scholar] [CrossRef]
- Lewiński, J.; Dranka, M.; Bury, W.; Śliwiński, W.; Justyniak, I.; Lipkowski, J. From Discrete Linear ZntBu2 Molecules to 1D Coordination Polymers and 2D Fabrics. J. Am. Chem. Soc. 2007, 129, 3096–3098. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Sokołowski, K.; Justyniak, I.; Kornowicz, A.; Fairen-Jimenez, D.; Friščić, T.; Lewiński, J. A Mechanochemical Strategy for IRMOF Assembly Based on Pre-Designed Oxo-Zinc Precursors. Chem. Commun. 2015, 51, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Nawrocki, J.; Terlecki, M.; Marynowski, W.; Lewiński, J. Facile Mechanosynthesis of the Archetypal Zn-Based Metal–Organic Frameworks. Inorg. Chem. 2018, 57, 13437–13442. [Google Scholar] [CrossRef]
- Nawrocki, J.; Prochowicz, D.; Wiśniewski, A.; Justyniak, I.; Goś, P.; Lewiński, J. Development of an SBU-Based Mechanochemical Approach for Drug-Loaded MOFs. Eur. J. Inorg. Chem. 2020, 2020, 796–800. [Google Scholar] [CrossRef]
- Lee, D.; Wolska-Pietkiewicz, M.; Badoni, S.; Grala, A.; Lewiński, J.; De Paëpe, G. Disclosing Interfaces of ZnO Nanocrystals Using Dynamic Nuclear Polarization: Sol-Gel versus Organometallic Approach. Angew. Chem. Int. Ed. 2019, 58, 17163–17168. [Google Scholar] [CrossRef]
- Krupiński, P.; Grala, A.; Wolska-Pietkiewicz, M.; Danowski, W.; Justyniak, I.; Lewiński, J. From Uncommon Ethylzinc Complexes Supported by Ureate Ligands to Water-Soluble ZnO Nanocrystals: A Mechanochemical Approach. ACS Sustain. Chem. Eng. 2021, 9, 1540–1549. [Google Scholar] [CrossRef]
- CrystalExplorer 17.5. Available online: crystalexplorer.scb.uwa.edu.au (accessed on 20 August 2021).
- Rai, V.K.; Srivastava, R.; Chauhan, G.; Saxena, K.; Bhardwaj, R.K.; Chand, S.; Kamalasanan, M.N. Synthesis and Electroluminescence Properties of Zinc(2,2′-Bipyridine)8-Hydroxyquinoline. Mater. Lett. 2008, 62, 2561–2563. [Google Scholar] [CrossRef]
- Deacon, G.; Phillips, R.J. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Li, S.; Lu, J.; Ma, H.; Yan, D.; Li, Z.; Qin, S.; Evans, D.G.; Duan, X. Luminous Ultrathin Films by the Ordered Micellar Assembly of Neutral Bis(8-Hydroxyquinolate)Zinc with Layered Double Hydroxides. J. Phys. Chem. C 2012, 116, 12836–12843. [Google Scholar] [CrossRef]
- Brinkmann, M.; Gadret, G.; Muccini, M.; Taliani, C.; Masciocchi, N.; Sironi, A. Correlation between Molecular Packing and Optical Properties in Different Crystalline Polymorphs and Amorphous Thin Films of Mer-Tris(8-Hydroxyquinoline)Aluminum(III). J. Am. Chem. Soc. 2000, 122, 5147–5157. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. CrysAlisPro. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 18 August 2021).
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Cryst. C 2015, C71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołtys-Brzostek, K.; Sokołowski, K.; Justyniak, I.; Leszczyński, M.K.; Olejnik-Fehér, N.; Lewiński, J. From a Well-Defined Organozinc Precursor to Diverse Luminescent Coordination Polymers Based on Zn(II)-Quinolinate Building Units Interconnected by Mixed Ligand Systems. Molecules 2021, 26, 7402. https://doi.org/10.3390/molecules26237402
Sołtys-Brzostek K, Sokołowski K, Justyniak I, Leszczyński MK, Olejnik-Fehér N, Lewiński J. From a Well-Defined Organozinc Precursor to Diverse Luminescent Coordination Polymers Based on Zn(II)-Quinolinate Building Units Interconnected by Mixed Ligand Systems. Molecules. 2021; 26(23):7402. https://doi.org/10.3390/molecules26237402
Chicago/Turabian StyleSołtys-Brzostek, Katarzyna, Kamil Sokołowski, Iwona Justyniak, Michał K. Leszczyński, Natalia Olejnik-Fehér, and Janusz Lewiński. 2021. "From a Well-Defined Organozinc Precursor to Diverse Luminescent Coordination Polymers Based on Zn(II)-Quinolinate Building Units Interconnected by Mixed Ligand Systems" Molecules 26, no. 23: 7402. https://doi.org/10.3390/molecules26237402