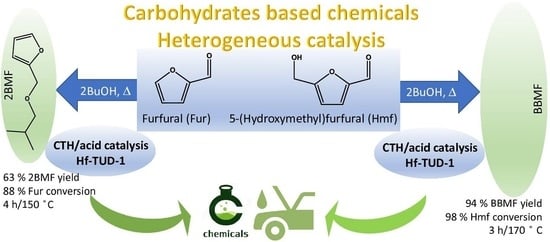
Catalytic Transfer Hydrogenation and Acid Reactions of Furfural and 5-(Hydroxymethyl)furfural over Hf-TUD-1 Type Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Studies
2.2.1. Furfural Conversion
Influence of the Reaction Temperature
Type of Solvent/H-Donor
Influence of the Initial Concentration of Furfural
Catalyst Dosage
Hafnium versus Zirconium Catalysts
Si/Hf Ratio of Hf-TUD-1 Materials
Literature Survey for Fur Conversion via CHT/Alcohol Strategies
2.2.2. 5-(Hydroxymethyl)furfural Conversion
2.2.3. Catalyst Stability
3. Materials and Methods
3.1. Synthesis of the Hf-TUD-1(x) Catalysts
3.2. Characterization of the Materials
3.3. Catalytic Tests
3.4. Kinetic Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- EPA-United States Environmental Protection Agency. Overview of Greenhouse Gases. 2019. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 20 October 2021).
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. 2021. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 20 October 2021).
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- International Furan Chemicals (IFC) B.V. Applications of Furfural. 2016. Available online: http://www.furan.com/furfural_applications_of_furfural.html (accessed on 20 October 2021).
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- International Furan Chemicals (IFC) B.V. Furfuryl Alcohol. 2016. Available online: http://www.furan.com/furfuryl_alcohol.html (accessed on 20 October 2021).
- Long, J.; Xu, Y.; Zhao, W.; Li, H.; Yang, S. Heterogeneous Catalytic Upgrading of Biofuranic Aldehydes to Alcohols. Front. Chem. 2019, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- International Furan Chemicals (IFC) B.V. International Furan Chemicals. 2016. Available online: http://www.furan.com/ifc.html (accessed on 20 October 2021).
- TransFuran Chemicals (TFC) B.V. TransFurans Chemicals. 2016. Available online: http://www.furan.com/tfc.html (accessed on 20 October 2021).
- Grand View Research (GVR). Furfuryl Alcohol Market Size, Share and Trends Analysis Report by Application (Resins, Solvent, Corrosion Inhibitor), by End Use (foundry, agriculture), by Region, and Segment Forecasts, 2020–2027. June 2020. Available online: https://www.grandviewresearch.com/industry-analysis/furfuryl-alcohol-market (accessed on 20 October 2021).
- International Furan Chemicals (IFC) B.V. Applications. 2016. Available online: http://www.furan.com/furfuryl_alcohol_applications.html (accessed on 20 October 2021).
- Chaffey, D.R.; Davies, T.E.; Taylor, S.H.; Graham, A.E. Ehterification Reactions of Furfuryl Alcohol in the presence of Orthoesters and Ketals: Application to the Synthesis of Furfuryl Ether Biofuels. ACS Sustain. Chem. Eng. 2018, 6, 4996–5002. [Google Scholar] [CrossRef]
- Haan, R.J.; Lange, J.-P. Gasoline Composition and Process for the Preparation of Alkylfurfuryl Ether. U.S. Patent 8,372,164 B2, 12 February 2013. [Google Scholar]
- Haan, R.J.; Lange, J.-P. Gasoline Composition and Process for the Preparation of Alkylfurfuryl Ether. E.P. Patent 22311832 B1, 29 September 2010. [Google Scholar]
- Padovan, D.; Al-Nayili, A.; Hammond, C. Bifunctional Lewis and Brönsted Acidic Zeolites Permit the Continuous Production of Bio-Renewable Furanic Ethers. Green Chem. 2017, 19, 2846–2854. [Google Scholar] [CrossRef]
- Natsir, T.A.; Shimazu, S. Fuels and Fuel Additives from Furfural Derivatives via Etherification and Formation of Methylfurans. Fuel Process. Technol. 2020, 200, 106308. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Daenen, L.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Furfuryl Ethyl Ether: Important Aging Flavor and New Market for the Storage Conditions of Beer. J. Agric. Food Chem. 2004, 52, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Haan, R.J.; Lange, J.-P. Gasoline Composition and Process for the Preparation of Alkylfurfuryl Ether. W.O. Patent 2009/077606 A2, 25 June 2009. [Google Scholar]
- Thoma, C.; Konnerth, J.; Sailer-Kronlachner, W.; Solt, P.; Rosenau, T.; van Herwijnen, H.W.G. Current Situation of the Challenging Scale-up Development of Hydroxymethylfurfural Production. ChemSusChem 2020, 13, 3544–3564. [Google Scholar] [CrossRef]
- R. C. News. AVALON Industries Wins New Swiss Research Project to Replace Carcinogenic Formaldehyde with Bio-Based, Non-Toxic 5-HMF. 2 February 2017. Available online: https://renewable-carbon.eu/news/avalon-industries-wins-new-swiss-research-project-to-replace-carcinogenic-formaldehyde-with-bio-based-non-toxic-5-hmf/ (accessed on 20 October 2021).
- Vyskocil, A.K.J. Verfahren zur Extraktion von Furfuralen aus Biomasse. D.E. Patent 102011053034 A1, 28 February 2013. [Google Scholar]
- Zhang, J.; Wang, T.; Tang, X.; Peng, L.; Wei, J.; Lin, L. Methods in the Syntheis and Conversion of 2,5-Bis-(Hydroxymethyl) furan from Bioderived 5-Hydroxymethylfurfural and its Great Potential in Polymeization. BioResources 2018, 13, 7137–7154. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent Advances in Catalytic Transformation of Biomass-Derived 5-Hydroxymethylfurfural Into the Innovative Fuels and Chemicals. Renew. Sust. Energ. Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Hu, L.; Xu, J.; Zhou, S.; He, A.; Tang, X.; Lin, L.; Xu, J.; Zhao, Y. Catalytic Advances in the Production and Application of Biomass-Derived 2,5-Dihydroxymethylfuran. ACS Catal. 2018, 8, 2959–2980. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Hu, H.; An, J.; Li, C. Efficient Conversion of 5-Hydroxymethylfurfural to High-Value Chemicals by Chemo-and Bio-Catalysis. RSC Adv. 2018, 8, 30875–30886. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Wei, J.; Ding, N.; Sun, Y.; Zeng, X.; Hu, L.; Liu, S.; Lei, T.; Lin, L. Chemoselective Hydrogenation of Biomass Derived 5-Hydroxymethylfurfural to Diols: Key Intermediates for Sustainable Chemicals, Materials and Fuels. Renew. Sust. Energ. Rev. 2017, 77, 287–296. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Bell, A.T.; Toste, F.D. Synthesis of Biomass-Derived Ethers for Use as Fuels and Lubrincants. ChemSusChem 2019, 12, 2835–2858. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Jiang, Y.; Wang, X.; He, A.; Xu, J.; Wu, Z. Recent Advances and Mechanistic insights on the Production of Biomass-Derived 2,5-Bis (Alkoxymethyl) Furans. Biomass Convers. Biorefin. 2020, 1–16. [Google Scholar] [CrossRef]
- Wei, J.; Wang, T.; Liu, H.; Li, M.; Tang, X.; Sun, Y.; Zeng, X.; Hu, L.; Lei, T.; Lin, T. High Efficient Reductive Etherification of 5-Hydroxymethylfurfural to 2,5-Bis(Alkoxymethyl)Furans as Biodiesel Components over Zr-SBA-15 Catalyst. Energy Technol. 2019, 7, 1801071. [Google Scholar] [CrossRef]
- Jae, J.; Mahmoud, E.; Lobo, R.F.; Vlachos, D.G. Cascade of Liquid-Phase Catalytic Transfer Hydrogenation and Etherification of 5-Hydroxymethylfurfural to Potential Biodiesel Components over Lewis Acid Zeolites. ChemCatChem 2014, 6, 508–513. [Google Scholar] [CrossRef]
- Zaccheria, F.; Bossola, F.; Scotti, N.; Evangelisti, C.; Dal Santo, V.; Ravasio, N. On Demand Production of Ethers or Alcohols from Furfural and HMF by Selecting the Composition of a Zr/Si Catalyst. Catal. Sci. Technol. 2020, 10, 7502–7511. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Recent Advances in Heterogeneous Catalytic Transfer Hydrogenation/Hydrogenolysis for Valorization of Biomass-Derived Furanic Compounds. Green Chem. 2021, 23, 670–688. [Google Scholar] [CrossRef]
- López-Asensio, R.; Jiménez Gómez, C.P.; Sancho, C.G.; Moreno-Tost, R.; Cecilia, J.A.; Maireles-Torres, P. Influence of Structure-Modifying Agents in the Synthesis of Zr-doped SBA-15 Silica and Their Use as Catalysts in the Furfural Hydrogenation to Obtain High Value-Added Products through the Meerwein-Ponndorf-Verley reduction. Int. J. Mol. Sci. 2019, 20, 828. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernández, B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911–1927. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Y.; Yang, S.; Wei, J.; He, L.; Peng, L.; Tang, X.; Ni, Y. Highly Selective Conversion of Furfural to Furfural Alcohol or Levulinate Ester in One Pot over ZrO2@SBA-15 and its Kinetic Behavior. ACS Sustain. Chem. Eng. 2020, 8, 5584–5594. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B.; Osatiashtiani, A.; Lee, A.F.; Wilson, K. ZrO2-SBA-15 Catalysts for the One-Pot Cascade Synthesi of GVL from Furfural. Catal. Sci. Technol. 2018, 8, 4485–4493. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Yu, J.; Gorte, R.J.; Mahmoud, E.; Vlachos, D.G.; Smith, M.A. The Effect of Oxide Activity on HMF Etherification. Catal. Sci. Technol. 2014, 4, 3074–3081. [Google Scholar] [CrossRef]
- Wei, J.; Cao, X.; Wang, T.; Liu, H.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Liu, S.; Lin, L. Catalytic Transfer Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Bis (Hydroxymethyl)furan over tunable Zr-based bimetallic catalysts. Catal. Sci. Technol. 2018, 8, 4474–4484. [Google Scholar] [CrossRef]
- Gao, L.; Li, G.; Sheng, Z.; Tang, Y.; Zhang, Y. Alkali-Metal-Ions Promoted Zr-Al-Beta Zeolite with High Selectivity and Resistance to Coking in the Conversion of Furfural Toward Furfural Alcohol. J. Catal. 2020, 389, 623–630. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Liu, Y.; Zhang, J.; He, L. Coupled Transfer Hydrogenation and Alcoholysis of Furfural to Yield Alkyl Levulinate over Multifunctional Zirconia-Zeolite-Supported Heteropoly Acid. Energy Fuels 2021, 35, 4182–4190. [Google Scholar] [CrossRef]
- Lewis, J.D.; Van de Vyver, S.; Crisci, A.J.; Gunther, W.R.; Michaelis, V.K.; Griffin, R.G.; Román-Leshkov, Y. A Continuous Flow Strategy for the Coupled Transfer Hydrogenation and Etherification of 5-(Hydroxymethyl)furfural using Lewis Acid Zeolties. ChemSusChem 2014, 7, 2255–2265. [Google Scholar] [CrossRef]
- Jansen, J.C.; Shan, Z.; Marchese, L.; Zhou, W.; de Puil, N.V.; Maschmeyer, T. A New Templating Method for Three-Dimensional Mesopore Networks. Chem. Commun. 2001, 8, 713–714. [Google Scholar] [CrossRef]
- Telalović, S.; Ramanathan, A.; Mul, G.; Hanefeld, U. TUD-1: Synthesis and Application of a Versatile Catalyst, Carrier, Material. J. Mater. Chem. 2010, 20, 642–658. [Google Scholar] [CrossRef] [Green Version]
- Dai, F.; Zhou, S.; Qin, X.; Liu, D.; Qi, H. Surfactant-Assisted Synthesis of Mesoporous Hafnium-Imidazoledicarboxylic Acid Hybrids for Highly Efficient Hydrogen Transfer of Biomass-Derived Carboxides. Mol. Catal. 2019, 479, 110611. [Google Scholar] [CrossRef]
- Sittiwong, J.; Boonmark, S.; Nunthakitgoson, W.; Maihom, T.; Wattanakit, C.; Limtrakul, J. Density Functional Investigation of the Conversion of Furfural to Furfuryl Alcohol by Reaction with i-Propanol over UiO-66 Metal Organic Framework. Inorg. Chem. 2021, 60, 4860–4868. [Google Scholar] [CrossRef]
- Koehle, M.; Lobo, R.F. Lewis Acidic Zeolite Beta Catalyst for the Meerwein-Ponndorf-Verley Reduction of Furfural. Catal. Sci. Technol. 2016, 6, 3018–3026. [Google Scholar] [CrossRef]
- Simons, C.; Hanefeld, U.; Arends, I.W.C.E.; Sheldon, R.A.; Maschmeyer, T. Nonconvent Anchoring of Assymetric Hydrogenation Catalysts on a New Mesoporous Aluminosilicate: Application and Solvent Effects. Chem. Eur. J. 2004, 10, 5829–5835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Bai, P.; Xu, B.; Yan, Z.-F. Synthesis of Mesoporous Alumina TUD-1 with High Thermostability. J. Porous Mater. 2006, 13, 245–250. [Google Scholar] [CrossRef]
- Shan, Z.; Jansen, J.C.; Zhou, W.; Maschmeyer, T. Al-TUD-1, stable mesoporous aluminas with high surface areas. Appl. Catal. A Gen. 2003, 254, 339–343. [Google Scholar] [CrossRef]
- Lima, S.; Antunes, M.M.; Fernandes, A.; Pillinger, M.; Ribeiro, M.F.; Valente, A.A. Acid Catalysed Conversion of Saccharides into Furanic Aldehydes in the Presence of Three-Dimensional Mesoporous Al-TUD-1. Molecules 2010, 15, 3863–3877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campelo, J.M.; Lafont, F.; Marinas, J.M. Pt/SAPO-5 and Pt-SAPO-11 as Catalysts for the Hydroisomerization and Hydrocracking of n-Octane. J. Chem.Soc. Faraday Trans. 1995, 91, 1551–1555. [Google Scholar] [CrossRef]
- Khabtou, S.; Lavalley, J.C. Quantitative Infrared Study of the Distinct Acidic Hydroxyl Groups Contained in Modified Y zeolites. Micropor. Mesopor. Mat. 1994, 3, 133. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, X.; Guo, X.; Wang, X. A High Resolution Solid State NMR Study on Nano-Structured HZSM-5 Zeolite. Catal. Lett. 1999, 60, 89–94. [Google Scholar] [CrossRef]
- Rhimi, B.; Mhamdi, M.; Kalevaru, V.N.; Martin, A. Synergy Between Vanadium and Molybdenum in Bimetallic ZSM-5 Supported Catalysts for Ethylene Ammoxidation. RSC Adv. 2016, 6, 65866–65878. [Google Scholar] [CrossRef]
- Ke, J.; Wang, I. Elucidation of the Role of Potassium Fluoride in the Chemical and Physical Nature of ZSM-5 Zeolite. Mater. Chem. Phys. 2001, 68, 157–165. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Cau-Dit-Coumes, C.; Chlique, C.; Dauzères, A.; Pochard, I. Magnesium and calcium silicates hydrates, Part I: Investigation of the Possible Magnesium Incorporation in Calcium Silicate Hydrate (C-S-H) and of the Calcium in Magnesium Silicate Hydrate (M-S-H). J. Appl. Geochem. 2018, 89, 229–242. [Google Scholar] [CrossRef]
- Chung, S.H.; Angelici, C.; Hinterding, S.O.M.; Weingarth, M.; Baldus, M.; Houben, K.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Role of Magnesium Silicates in Wet-Kneaded Silica-Magnesia Catalysts for the Lebedev Ethanol-to-Butadiene process. ACS Catal. 2016, 6, 4034–4045. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Fernandes, A.; Neri, F.; Silva, C.M.; Rocha, S.M.; Ribeiro, M.F.; et al. One-Pot Conversion of Furfural to Useful Bio-Products in the Presence of a Sn, Al-Containing Zeolite Beta Catalyst Prepared via Post-Synthesis Route. J. Catal. 2015, 329, 522–537. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated Reduction and Acid-Catalysed Conversion of Furfural in Alcohol Medium Using Zr, Al-Containing Ordered Micro/Mesoporous Silicates. Appl. Catal. B Environ. 2016, 182, 485–503. [Google Scholar] [CrossRef] [Green Version]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of g-Valerolactone from Furfural by a Single-Step Process Using Sn-Al-Beta Zeolites: Optimizing the Catalyst Acid Properties and Process Conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Winoto, H.P.; Fikri, Z.A.; Ha, J.-M.; Park, Y.-K.; Lee, H.; Suh, D.J.; Jae, J. Heteropolyacid Supported on Zr-Beta Zeolite as an Active Catalyst for One-Pot Transformation of Furfural to γ-Valerolactone. Appl. Catal. B Environ. 2019, 241, 588–597. [Google Scholar] [CrossRef]
- Antunes, M.M.; Neves, P.; Fernandes, A.; Lima, S.; Silva, A.F.; Ribeiro, M.F.; Silva, C.M.; Pillinger, M.; Valente, A.A. Bulk and Composite Catalysts Combining BEA Topology and Mesoporosity for the Valorisation of Furfural. Catal. Sci. Technol. 2016, 6, 7812–7829. [Google Scholar] [CrossRef]
- Mao, W.; Liu, J.; Yin, B.; Kong, D.; Miao, S.; Wang, F. Transfer Hydrogenation of Furfural Catalysed by Multi-Centers Collaborative Ni-Based Catalyst and Kinetic Research. Appl. Catal. A Gen. 2021, 623, 118247. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Bisio, C.; Vittoni, C.; Ravasio, N. Switching Selectivity in the Hydrogen Transfer Reduction of Furfural. ChemistrySelect 2018, 3, 8344–8348. [Google Scholar] [CrossRef]
- Rao, B.S.; Kumari, P.K.; Koley, P.; Tardio, J.; Lingaiah, N. One-Pot Selective Conversion of Furfural to g-Valerolactone over Zirconium Containing Heteropolytungstate Supported on b Zeolite Catalyst. Mol. Catal. 2019, 466, 52–59. [Google Scholar]
- Peng, Q.; Wang, H.; Xia, Y.; Liu, X. One-Pot Conversion of Furfural to gamma-Valerolactone in the Presence of Multifunctional Zirconium Alizarin Red S Hybrid. Appl. Catal. A Gen. 2021, 621, 118203. [Google Scholar] [CrossRef]
- Tang, K.; Xie, S.; Cofield, G.R.; Yang, X.; Tian, E.; Lin, H. Catalyst Transfer Hydrogenation of Furfural for the Production of Ethyl Levulinate: Interplay of Lewis and Brönsted Acidities. Energy Technol. 2018, 6, 1826–1831. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic Transfer Hydrogenation of Butyl Levulinate to g-Valerolactone Over Zirconium Phosphates with Adjustable Lewis and Brönsted Acid Sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Van der Waal, J.C.; Kunkeler, P.J.; Tan, K.; van Bekkum, H. A Selective Catalyst for the Gas-Phase Meerwein-Ponndorf-Verley and Oppenauer Reactions. J. Catal. 1998, 173, 74–83. [Google Scholar] [CrossRef]
- Li, M.; Wei, J.; Yan, G.; Liu, H.; Tang, X.; Sun, Y.; Zeng, X.; Lei, T.; Lin, L. Cascade Conversion of Furfural to Fuel Bioadittive Ethyl Levulinate Over Bifuncational Zirconium-Based Catalysts. Renew. Energy 2020, 147, 916–923. [Google Scholar] [CrossRef]
- Ma, M.; Hou, P.; Zhang, P.; Cao, J.; Liu, H.; Yue, H.; Tian, G.; Feng, S. Magnetic Fe3O4 Nanoparticles as Easily Separable Catalysts Transfer Hydrogenation of Biomass-Derived Furfural to Furfuryl Alcohol. Appl. Catal. A Gen. 2020, 602, 117709. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Riisager, A.; Yang, S. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol with Recyclable Al-Zr@Fe Mixed Oxides. ChemCatChem 2018, 10, 430–438. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Fang, Z.; Smith, R.L. Efficient Catalytic Transfer Hydrogenation of Biomass-Based Furfural to Furfuryl Alcohol with Recycable Hf-Phenylphosphonate Nanohybrids. Catal. Today 2019, 319, 84–92. [Google Scholar] [CrossRef]
- Wang, M.; Peng, L.; Gao, X.; He, L.; Zhang, J. Efficient One-Pot Synthesis of Alkyl Levulinate from Xylose with an Integrated Dehydration/Transfer Hydrogenation/Alcoholysis Process. Sustain. Energy Fuels 2020, 4, 1383–1395. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Roslan, I.I.; Jaenicke, S.; Chuah, G.-K.; Combo, A. Zr-HY and Al-Hy Zeolite Catalysts for the One-Pot Cascade Transformation of Biomass-Derived to g-Valerolactone. J. Catal. 2019, 375, 56–67. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Song, W.-C.; Li, Y.; Yang, E.-C. One-Pot Transformation of Furfural into g-Valerolactone Catalyzed by a Hierarchical Hf-Al-USY Zeolite with Balanced Lewis and Brönsted Acid Sites. Sustain. Energy Fuels 2021, 5, 4724–4735. [Google Scholar] [CrossRef]
- García-Sancho, C.; Jiménez-Gómez, C.P.; Viar-Antuñano, N.; Cecilia, J.A.; Moreno-Tost, R.; Mérida-Robles, J.M.; Requies, J.; Maireles-Torres, P. Evaluation of the ZrO2/Al2O3 System as Catalysts in the Catalytic Transfer Hydrogenation of Furfural to Obtain Furfuryl Alcohol. Appl. Catal. A Gen. 2021, 609, 117905. [Google Scholar] [CrossRef]
- Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Soares, O.S.G.P.; Pereira, M.F.R.; Kubička, D.; Freire, C. Catalytic Transfer Hydrogenation of Furfural Over Co3O4-Al2O4 Hydrotalcite-Derived Catalyst. ChemCatChem 2020, 12, 1467–1475. [Google Scholar] [CrossRef]
- Shinde, S.; Rode, C. Cascade Reductive Etherification of Bioderived Aldehydes Over Zr-Based Catalysts. ChemSusChem 2017, 10, 4090–4101. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Efficient Valorization of Biomass-Derived Furfural to Fuel Bio-Additive Over Aluminium Phosphate. Appl. Catal. B Environ. 2021, 298, 120575. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, R. Zirconium Phosphate Catalyzed Transformations of Biomass-Derived Furfural to Renewable Chemicals. ACS Sustain. Chem. Eng. 2020, 8, 9497–9506. [Google Scholar] [CrossRef]
- Naguib, M.; Tang, W.; Browning, K.L.; Veith, G.M.; Maliekkal, V.; Neurock, M.; Villa, A. Catalytic Activity of Ti-Based MXenes For the Hydrogenation of Furfural. ChemCatChem 2020, 12, 5733–5742. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Martin, N.; Vlachos, D.G. Effect of Hydrogen Donor on Liquid Phase Catalytic Transfer Hydrogenation of Furfural Over a Ru/RuO2/C Catalyst. J. Mol. Catal. A Chem. 2014, 392, 223–228. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Gogoi, P.; Lucas, N.; Chilukuri, S.V. Novel Ru Nanoparticles Catalysts For Catalytic Transfer Hydrogenation of Biomass-Derived Furanic Compounds. Sustain. Energy Fuels 2020, 4, 3654–3667. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Popovych, N.O.; Kyriienko, P.I.; Millot, Y.; Valentin, L.; Gurgul, J.; Socha, R.P.; Żukrowski, J.; Soloviev, S.O.; Dzwigaj, S. Sn-BEA Zeolites Prepared by Two Step PostSynthesis Method: Physicochemical Properties and Catalytic Activity in Processes Based on MPV Reduction. Micropor. Mesopor. Mat. 2018, 268, 178–188. [Google Scholar] [CrossRef]
- Dell, L.A.O.; Gunawidjaja, P.N.; Holland, M.A.; Mountjoy, G.; Pickup, D.M.; Newport, R.J.; Smith, M.E. Characterisation of Sol-Gel prepared (HfO2)x(SiO2)1-x (X=0.1, 0.2 and 0.4) by 1H, 13C, 17O and 29Si MAS NMR, FTIR and TGA. Solid State Nucl. Magn. Reson. 2008, 33, 16–24. [Google Scholar]
- Nandiyanto, A.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Lynch, C.T.; Mazdiyasni, K.S.; Smith, J.S.; Crawford, W.J. Infrared Spectra of Transition Metal Alkoxides. Anal. Chem. 1964, 36, 2332–2337. [Google Scholar] [CrossRef]
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly Efficient Conversion of Glycerol ato Solketal Over Heterogeneous Lewis Acid Catalysts. Green Chem. 2012, 14, 1611–1619. [Google Scholar] [CrossRef]











| Sample | Si/Hf | SBET (m2 g−1) | Vp (cm3 g−1) | Dp (nm) | Amount of Acid Sites 1 (µmol g−1) | L/B | ||
|---|---|---|---|---|---|---|---|---|
| L | B | L + B | ||||||
| Hf-TUD-75 | 67 ± 4 | 490 | 2.4 | 11–18 | 93 | 12 | 105 | 7.6 |
| Hf-TUD-50 | 44 ± 5 | 660 | 2.0 | 6−10 | 120 | 10 | 130 | 12.0 |
| Hf-TUD-25 | 23 ± 5 | 721 | 2.0 | 6–10 | 163 | 18 | 181 | 9.1 |
| Catalyst | T (°C) | t (h) | Solv. | [Fur]0 | Cat/Fur (m/m) | C (%) | YAMF (%) | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| OMSi | |||||||||
| 1 | Hf-TUD-1(75) | 150 | 4 | 2BuOH | 0.2 | 1.0 | 88 | 63 | - |
| 2 | Hf-TUD-1(50) | 150 | 8 | 2BuOH | 0.2 | 0.25 | 84 | 65 | - |
| 3 | Zr-TUD-1(25) | 120 | 7 | 2BuOH | 0.45 | 0.6 | 70 | 55 | [62] |
| 4 | Zr-MCM-41 | 120 | 3 | 2PrOH | 1.31 | 0.21 | 44 | 20 | [42] |
| 5 | HPMo(20)/Zr-MCM-41 | 150 | 24 | 2PrOH | 0.05 | 8.33 | 76 | 20 | [43] |
| 6 | Zr-SBA-15 | 130 | 6 | 2PrOH | 0.27 | 1.0 | 100 | 86 | [37] |
| 7 | Zr-SBA-15-RT | 130 | 3 | 2PrOH | 0.26 | 1.0 | 82 | 61 | [36] |
| 8 | ZrO2/SBA-15, 17wt%Zr | 150 | 2.5 | 2PrOH | 0.26 | 0.4 | 100 | 70 | [39] |
| Zeolitic materials | |||||||||
| 9 | ZrdeAl-Beta | 120 | 3 | 2PrOH | 1.31 | 0.21 | 50 | 41 | [42] |
| 10 | ZrAl-Beta-n | 150 | 7 | 2BuOH | 0.45 | 0.32 | 95 | 35 | [65] |
| 11 | ZrAl-Beta-n | 120 | 7 | 2BuOH | 0.45 | 0.6 | 85 | 55 | [62] |
| 12 | MP-ZrAl-Beta-m | 150 | 5 | 2BuOH | 0.45 | 0.6 | 99 | 88 | [65] |
| 13 | (Sn)SSIE-Beta | 120 | 5 | 2BuOH | 0.45 | 0.6 | 86 | 58 | [61] |
| 14 | Zr-Beta-75-75 | nm | 0.33 | 2PeOH | 0.2 | 0.52 | 24 | 17 | [78] |
| 15 | Zr-HY-15-5 | nm | 0.33 | 2PeOH | 0.2 | 0.52 | 50 | 36 | [78] |
| 16 | 2Hf-Al-USY-8 | 140 | 12 | 2PrOH | 0.1 | 1.04 | 100 | 41 | [79] |
| Metal oxides | |||||||||
| 17 | ZrO2 | 130 | 6 | 2PrOH | 0.26 | 1.0 | 70 | 17 | [80] |
| 18 | Co3O4-Al2O3 | 180 | 6 | 2PrOH | 0.12 | 0.43 | 92 | 9 | [81] |
| 19 | ~70wt%ZrO2/SiO2 | 120 | 4 | 2BuOH | 0.13 | 1.0 | 100 | 95 | [34] |
| 20 | 3.5wt%SiO2/ZrO2 | 140 | 1 | 2PrOH | 0.07 | 0.42 | 56 | 49 | [67] |
| 21 | 16%Cu/SiO2 | 180 | 5 | 2PrOH | 0.07 | 0.42 | 72 | 36 | [67] |
| Others | |||||||||
| 22 | Zr-Mt+ZrO(OH)2 2 | 100 | 2 | 2BuOH | 0.4 | 0.8 | 100 | 69 | [82] |
| 23 | APO-5 | 120 | 48 | 2PrOH | 0.2 | 1.04 | 90 | 55 | [83] |
| 24 | Zr-PO4 | 120 | 6 | 2PrOH | 0.14 | 3.7 | 95 | 24 | [84] |
| 25 | Zr-AlBeta/TUD-1 | 150 | 2 | 2BuOH | 0.45 | 0.6 | 86 | 60 | [65] |
| 26 | Ti3C2Tz MXene 3 | 180 | 48 | 2PrOH | 0.3 | 50 | 62 | 13 | [85] |
| Catalyst | T (°C) | t (h) | Solv. | [Hmf]0 | Cat/Hmf (m/m) | C (%) | YBAMF (%) | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| OMSi | |||||||||
| 1 | Hf-TUD-1(75) | 150 | 21 | 2BuOH | 0.2 | 1 | 96 | 93 | - |
| 2 | Hf-TUD-1(50) | 150 | 3 | 2BuOH | 0.2 | 1 | 80 | 79 | - |
| 3 | Hf-TUD-1(50) | 170 | 3 | 2BuOH | 0.2 | 1 | 98 | 94 | - |
| 4 | 10%ZrO2/SBA-15 | 140 | nm | 2PrOH | 0.08 | 0.1 | 12 | 8 | [40] |
| 5 | 10%ZrO2/SBA-15 | 180 | nm | 2PrOH | 0.08 | 0.4 | 78 | 65 | [40] |
| 6 | Zr-SBA-15 | 150 | 2.5 | 2PrOH | 0.33 | 0.5 | 100 | 24 | [41] |
| 7 | Zr-SBA-15 | 180 | 4 | 2PrOH | 0.33 | 0.5 | 100 | 81 | [41] |
| 8 | Zr-AlSBA-15 | 120 | 2.5 | 2PrOH | 0.33 | 0.5 | 17 | 5 | [41] |
| 9 | Zr-SBA-15-UH | 150 | 3 | 2PrOH | 0.06 | 0.5 | 96 | 91 | [32] |
| 10 | Zr-SBA-15-UH | 150 | 4 | 2BuOH | 0.06 | 0.5 | 82 | 4 | [32] |
| 11 | Zr-MCM-41 | 120 | 24 | EtOH | 0.06 | 0.03 2 | 41 | 8 | [44] |
| Zeolitic materials | |||||||||
| 12 | Sn-Beta | 150 | 6 | 2PrOH | 0.08 | 0.5 | 68 | 56 | [33] |
| 13 | Sn-Beta | 180 | 6 | 2PrOH | 0.08 | 0.5 | 82 | 71 | [33] |
| 14 | Sn-Beta | 180 | 6 | 2BuOH | 0.08 | 0.5 | 85 | 71 | [33] |
| 15 | Hf-Beta | 120 | 24 | EtOH | 0.06 | 0.03 2 | 87 | 67 | [44] |
| 16 | Zr-Beta | 120 | 24 | EtOH | 0.06 | 0.03 2 | 81 | 54 | [44] |
| 17 | Sn-Beta | 120 | 24 | EtOH | 0.06 | 0.03 2 | 69 | 41 | [44] |
| 18 | Hf-Beta | 120 | 1 | 1BuOH | 0.06 | 0.03 2 | 84 | 22 | [44] |
| 19 | Sn-Beta | 140 | nm | 2PrOH | 0.08 | 0.1 | 13 | 11 | [40] |
| 20 | Sn-Beta | 180 | nm | 2PrOH | 0.08 | 0.4 | 70 | 61 | [40] |
| 21 | Sn-SiBeta | 95 | 8 | 2BuOH | 0.27 | 0.32 | 15 | 15 | [89] |
| 22 | Hf-Beta | 120 | 1 | 2BuOH | 0.06 | 0.03 2 | 93 | 81 | [44] |
| Others | |||||||||
| 23 | ~70wt%ZrO2/SiO2 | 120 | 7 | 2BuOH | 0.1 | 1 | 98 | 96 | [34] |
| 24 | Zr-Mt+ZrO(OH)2 3 | 150 | 1 | 2BuOH | 0.4 | 0.8 | 100 | 96 | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, M.M.; Silva, A.F.; Bernardino, C.D.; Fernandes, A.; Ribeiro, F.; Valente, A.A. Catalytic Transfer Hydrogenation and Acid Reactions of Furfural and 5-(Hydroxymethyl)furfural over Hf-TUD-1 Type Catalysts. Molecules 2021, 26, 7203. https://doi.org/10.3390/molecules26237203
Antunes MM, Silva AF, Bernardino CD, Fernandes A, Ribeiro F, Valente AA. Catalytic Transfer Hydrogenation and Acid Reactions of Furfural and 5-(Hydroxymethyl)furfural over Hf-TUD-1 Type Catalysts. Molecules. 2021; 26(23):7203. https://doi.org/10.3390/molecules26237203
Chicago/Turabian StyleAntunes, Margarida M., Andreia F. Silva, Carolina D. Bernardino, Auguste Fernandes, Filipa Ribeiro, and Anabela A. Valente. 2021. "Catalytic Transfer Hydrogenation and Acid Reactions of Furfural and 5-(Hydroxymethyl)furfural over Hf-TUD-1 Type Catalysts" Molecules 26, no. 23: 7203. https://doi.org/10.3390/molecules26237203