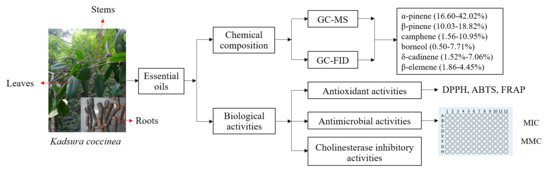
Chemical Composition and Biological Activities of Essential Oils from the Leaves, Stems, and Roots of Kadsura coccinea
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of K. coccinea Essential Oils
2.2. Antioxidant Activity of K. coccinea Essential Oils
2.3. Antimicrobial Activity of K. coccinea Essential Oils
2.4. Cholinesterase Inhibition Activity of K. coccinea Essential Oils
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals
4.3. Extraction of Essential Oils
4.4. GC-MS and GC-FID Analysis
4.5. Antioxidant Activities
4.5.1. DPPH Radical Scavenging Assay
4.5.2. ABTS Cation Radical Scavenging Assay
4.5.3. Ferric Reducing Antioxidant Power Assay
4.6. Antimicrobial Activities
4.7. Cholinesterase Inhibition Activities
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, L.; Su, K.D.; Wei, X.C.; Zhang, J.; Li, H.R. Chemical constituents of Kadsura coccinea. Chem. Nat. Compd. 2018, 54, 242–244. [Google Scholar] [CrossRef]
- Li, H.R.; Feng, Y.L.; Yang, Z.G.; Wang, J.; Daikonya, A.; Kitanaka, S.; Xu, L.Z.; Yang, S.L. New lignans from Kadsura coccinea and their nitric oxide inhibitory activities. Chem. Pharm. Bull. 2006, 54, 1022–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, N.K.; Van Thanh, B.; Van Kiem, P.; Van Minh, C.; Cuong, N.X.; Nhiem, N.X.; Huong, H.T.; Anh, H.T.; Park, E.J.; Sohn, D.H.; et al. Dibenzocyclooctadiene Lignans and Lanostane Derivatives from the Roots of Kadsura coccinea and their Protective Effects on Primary Rat Hepatocyte Injury Induced by t-Butyl Hydroperoxide. Planta Med. 2009, 75, 1253–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Li, L.; Wang, Q.; Ye, Y.; Fan, J.; Li, H.-X.; Kitanaka, S.; Li, H.-R. Dibenzocyclooctadiene lignans from Kadsura coccinea. J. Asian Nat. Prod. Res. 2012, 14, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.H.; Cheng, L.; He, Q.Q.; Kong, L.Y. A lignin glycoside and a nortriterpenoid from Kadsura coccinea. Chin. J. Nat. Med. 2014, 12, 782–785. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Daniyal, M.; Yu, H.; Xie, Q.; Li, B.; Jian, Y.; Man, R.; Wang, S.; Zhou, X.; et al. New Lignans from roots of Kadsura coccinea. Fitoterapia 2019, 139, 104368. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Miyata, S.; Kitanaka, S. Kadsuracoccinic acids A-C, ring-A seco-lanostane triterpenes from Kadsura coccinea and their effects on embryonic cell division of Xenopus laevis. J. Nat. Prod. 2008, 71, 739–741. [Google Scholar] [CrossRef]
- Gao, X.M.; Pu, J.X.; Xiao, W.L.; Huang, S.X.; Lou, L.G.; Sun, H.D. Kadcoccilactones K-R, triterpenoids from Kadsura coccinea. Tetrahedron 2008, 64, 11673–11679. [Google Scholar] [CrossRef]
- Wang, N.; Li, Z.L.; Li, D.Y.; Pei, Y.H.; Hua, H.M. Five New Triterpenoids from the Roots of Kadsura coccinea. Helv. Chim. Acta 2009, 92, 1413–1418. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Q.J.; Jin, Y.S.; Feng, C.W.; Chen, H.S. Two New Triterpenoid Acids from Kadsura coccinea. Arch. Pharmacal Res. 2010, 33, 1933–1936. [Google Scholar] [CrossRef]
- Hu, Z.X.; Li, X.N.; Shi, Y.M.; Wang, W.G.; Du, X.; Li, Y.; Zhang, Y.H.; Pu, J.X.; Sun, H.D. Lanostane-type triterpenoids from Kadsura coccinea. Tetrahedron 2017, 73, 2931–2937. [Google Scholar] [CrossRef]
- Xu, H.C.; Hu, K.; Sun, H.D.; Puno, P.T. Four 14(1312)-Abeolanostane Triterpenoids with 6/6/5/6-Fused Ring System from the Roots of Kadsura coccinea. Nat. Prod. Bioprospecting 2019, 9, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Xie, C.; Wang, H.; Jin, D.Q.; Xu, J.; Guo, Y.; Ma, Y. Lignans from the roots of Kadsura coccinea and their inhibitory activities on LPS-induced NO production. Phytochem. Lett. 2014, 9, 158–162. [Google Scholar] [CrossRef]
- Sun, J.; Yao, J.; Huang, S.; Long, X.; Wang, J.; Garcia-Garcia, E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) AC Smith. Food Chem. 2009, 117, 276–281. [Google Scholar] [CrossRef]
- Sritalahareuthai, V.; Temviriyanukul, P.; On-nom, N.; Charoenkiatkul, S.; Suttisansanee, U. Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Foods 2020, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Hong-Loan, N.; Thu-Huyen Thi, T.; Thuong Thien, P.; Tuan-Nghia, P. In vitro Inhibitory Effect of Lanostane Triterpenoids of Kadsura coccinea on the Human Immunodeficiency Virus Type-1 Protease. Indian J. Pharm. Sci. 2018, 80, 755–761. [Google Scholar]
- Zhang, X.Y.; Wei, Z.J.; Qiao, W.L.; Sun, X.M.; Jin, Z.L.; Gong, W.; Jia, B.X. Discovery of cyclooxygenase-2 inhibitors from Kadsura coccinea by affinity ultrafiltration mass spectrometry and the anti-inflammatory activity. Fitoterapia 2021, 151, 104872. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Z.; Yang, Y.P.; Cheng, S.W.; Cao, L.; Xie, Q.L.; Wang, M.Y.; Li, B.; Jian, Y.Q.; Liu, B.; Peng, C.Y.; et al. Heilaohuguosus A-S from the fruits of Kadsura coccinea and their hepatoprotective activity. Phytochemistry 2021, 184, 112678. [Google Scholar] [CrossRef]
- Huang, S.Z.; Duan, L.P.; Wang, H.; Mei, W.L.; Dai, H.F. Two New AChE Inhibitors Isolated from Li Folk Herb Heilaohu “Kadsura coccinea” Stems. Molecules 2019, 24, 3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi Hee, W.; Duc Hung, N.; Choi, J.S.; Park, S.E.; Phuong Thien, T.; Byung Sun, M.; Duc Dat, L. Chemical constituents from the roots of Kadsura coccinea with their protein tyrosine phosphatase 1B and acetylcholinesterase inhibitory activities. Arch. Pharmacal Res. 2020, 43, 204–213. [Google Scholar]
- Dai, D.N.; Thanh, B.V.; Anh, L.D.N.; Ban, N.K.; Thang, T.D.; Ogunwande, I.A. Composition of Stem Bark Essential Oils of Three Vietnamese Essential Oils of Three Vietnamese Species of Kadsura (Schisandraceae). Rec. Nat. Prod. 2015, 9, 386–393. [Google Scholar]
- Rehman, J.U.; Wang, M.; Yang, Y.; Liu, Y.; Li, B.; Qin, Y.; Wang, W.; Chittiboyina, A.G.; Khan, I.A. Toxicity of Kadsura coccinea (Lem.) A. C. Sm. Essential Oil to the Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae). Insects 2019, 10, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Gao, J.F. Volatile components and their antioxidant activities in different parts of Kadsura coccinea (in Chinese). Guihaia 2018, 38, 943–952. [Google Scholar]
- Zuo, Y.; Wang, S.; Shao, F.; He, T.; Tan, Y.; Yang, C. Analysis of volatile components of essential oil from different organs of Kadsura coccinea by GC-MS (in Chinese). Non-Wood For. Res. 2021, 39, 175–185. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. /Fundam. Mol. Mech. Mutagenesis 2003, 523, 9–20. [Google Scholar] [CrossRef]
- Asghari, B.; Zengin, G.; Bahadori, M.B.; Abbas-Mohammadi, M.; Dinparast, L. Amylase, glucosidase, tyrosinase, and cholinesterases inhibitory, antioxidant effects, and GC–MS analysis of wild mint (Mentha longifolia var. calliantha) essential oil: A natural remedy. Eur. J. Integr. Med. 2018, 22, 44–49. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm Pharm. 2010, 62, 1037–1044. [Google Scholar] [CrossRef]
- Dai, D.N.; Tuan, N.N.; Thang, T.D.; Ogunwande, I.A. Kadsura oblongifolia Merr. leaves, stem bark and root bark essential oils. J. Essent. Oil Res. 2014, 26, 372–376. [Google Scholar] [CrossRef]
- Li, H.Q.; Bai, C.Q.; Chu, S.S.; Zhou, L.G.; Du, S.S.; Liu, Z.L.; Liu, Q.Z. Chemical composition and toxicities of the essential oil derived from Kadsura heteroclita stems against Sitophilus zeamais and Meloidogyne incognita. J. Med. Plant. Res. 2011, 5, 4943–4948. [Google Scholar]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. δ-Cadinene, Calarene and δ-4-Carene from Kadsura heteroclita essential oil as novel larvicides against Malaria, dengue and Filariasis mosquitoes. Comb. Chem. High. Throughput Screen. 2016, 19, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Su, J.Y.; Li, L.; Li, B.; Li, W. A new source of natural D-borneol and its characteristic. J. Med. Plants Res. 2011, 5, 3440–3447. [Google Scholar]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Gois Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; GómezSerranillos, M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Liu, Y.; Liu, S.; Tan, Y. Diblock copolymer containing bioinspired borneol and dopamine moieties: Synthesis and antibacterial coating applications. Polymer 2017, 116, 314–323. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crop. Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Rajaofera, M.J.N.; Jin, P.F.; Fan, Y.M.; Sun, Q.Q.; Huang, W.K.; Wang, W.B.; Shen, H.Y.; Zhang, S.; Lin, C.H.; Liu, W.B.; et al. Antifungal activity of the bioactive substance from Bacillus atrophaeus strain HAB-5 and its toxicity assessment on Danio rerio. Pestic Biochem Physiol. 2018, 147, 153–161. [Google Scholar] [CrossRef]
- Sella, S.R.; Vandenberghe, L.P.; Soccol, C.R. Bacillus atrophaeus: Main characteristics and biotechnological applications-a review. Crit Rev. Biotechnol. 2015, 35, 533–545. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yamafuji, C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J. Agric. Food. Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food. Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food. Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.P.; Zhao, H.P.; Zhou, H.L.; Zhang, Y. Chemical composition and antimicrobial activity of the essential oil from the aerial part of Dictamnus dasycarpus Turcz. Ind. Crop. Prod. 2019, 140, 111713. [Google Scholar] [CrossRef]
- Baltzar, B.K. Minimal Inhibitory Concentration (MIC). Available online: https://dx.doi.org/10.17504/protocols.io.gpwbvpe (accessed on 14 March 2017).
- Sun, Q.Y.; Yang, F.M. Comparative study on microassays for screening acetylcholinesterase inhibitor (in Chinese). Chin. Pharmacol. Bull. 2008, 24, 1387–1392. [Google Scholar]
- Yang, F.M.; Sun, Q.Y. Study on microassay for screening butyrylcholinesterase inhibitors (in Chinese). Chin. Pharmacol. Bull. 2009, 25, 690–692. [Google Scholar]
| No. | Compound | RI Calc. | RI Lit. | Identification | Relative Percentage (%) | ||
|---|---|---|---|---|---|---|---|
| Leaf Oil | Stem Oil | Root Oil | |||||
| 1 | tricyclene | 1011 | 1012 | RI; MS | 0.08 ± 0.01 | 0.27 ± 0.005 | 0.63 ± 0.02 |
| 2 | α-pinene | 1026 | 1025 | RI; MS | 42.02 ± 0.10 | 41.79 ± 0.83 | 16.60 ± 0.34 |
| 3 | α-thujene | 1028 | 1027 | RI; MS | nd | nd | 2.07 ± 0.13 |
| 4 | α-fenchene | 1058 | 1061 | RI; MS | 0.07 ± 0.00 | 0.09 ± 0.001 | 0.20 ± 0.01 |
| 5 | camphene | 1068 | 1068 | RI; MS | 1.56 ± 0.02 | 4.43 ± 0.13 | 10.95 ± 0.15 |
| 6 | β-pinene | 1113 | 1110 | RI; MS | 18.82 ± 0.08 | 18.71 ± 0.41 | 10.03 ± 0.20 |
| 7 | sabinene | 1123 | 1122 | RI; MS | 0.80 ± 0.003 | 1.10 ± 0.03 | 1.01 ± 0.02 |
| 8 | 3-carene | 1150 | 1147 | RI; MS | nd | 3.39 ± 0.08 | 2.43 ± 0.05 |
| 9 | myrcene | 1164 | 1161 | RI; MS | 2.93 ± 0.02 | 2.89 ± 0.07 | 1.87 ± 0.03 |
| 10 | α-phellandrene | 1166 | 1168 | RI; MS | 0.20 ± 0.01 | 0.26 ± 0.01 | 0.12 ± 0.01 |
| 11 | α-terpinene | 1182 | 1178 | RI; MS | 0.60 ± 0.01 | 0.30 ± 0.01 | 0.19 ± 0.01 |
| 12 | d-limonene | 1202 | 1198 | RI; MS | 1.69 ± 0.01 | 1.31 ± 0.04 | 1.43 ± 0.02 |
| 13 | β-phellandrene | 1211 | 1209 | RI; MS | 2.05 ± 0.02 | 1.99 ± 0.05 | nd |
| 14 | eucalyptol | 1213 | 1211 | RI; MS | nd | nd | 2.23 ± 0.03 |
| 15 | 2-pentyl furan | 1234 | 1235 | RI; MS | nd | 0.11 ± 0.01 | nd |
| 16 | γ-terpinene | 1247 | 1245 | RI; MS | 1.01 ± 0.01 | 0.54 ± 0.04 | 0.33 ± 0.01 |
| 17 | (E)-β-ocimene | 1253 | 1250 | RI; MS | 0.27 ± 0.01 | 0.08 ± 0.03 | nd |
| 18 | p-cymene | 1273 | 1270 | RI; MS | 0.08 ± 0.01 | 0.38 ± 0.005 | 0.38 ± 0.01 |
| 19 | terpinolene | 1285 | 1282 | RI; MS | 0.66 ± 0.01 | 0.27 ± 0.01 | 0.14 ± 0.00 |
| 20 | α-cubebene | 1459 | 1460 | RI; MS | nd | 0.15 ± 0.01 | 0.34 ± 0.01 |
| 21 | cyclosativene | 1482 | 1483 | RI; MS | nd | 0.11 ± 0.001 | 0.13 ± 0.01 |
| 22 | α-copaene | 1493 | 1491 | RI; MS | 0.89 ± 0.01 | 2.29 ± 0.04 | 3.03 ± 0.04 |
| 23 | β-cubebene | 1540 | 1542 | RI; MS | nd | 0.06 ± 0.00 | 0.18 ± 0.01 |
| 24 | linalool | 1547 | 1543 | RI; MS | 0.07 ± 0.001 | nd | nd |
| 25 | isoledene | 1553 | 1559 | RI; MS | nd | nd | 0.13 ± 0.01 |
| 26 | bornyl acetate | 1583 | 1579 | RI; MS | 0.14 ± 0.002 | 0.46 ± 0.02 | 0.60 ± 0.05 |
| 27 | fenchol | 1584 | 1575 | RI; MS | 0.39 ± 0.01 | nd | nd |
| 28 | β-elemene | 1593 | 1591 | RI; MS | 4.45 ± 0.05 | 2.04 ± 0.07 | 1.86 ± 0.04 |
| 29 | β-caryophyllene | 1600 | 1598 | RI; MS | 2.87 ± 0.04 | 2.71 ± 0.10 | 3.08 ± 0.09 |
| 30 | terpinen-4-ol | 1605 | 1601 | RI; MS | 1.69 ± 0.03 | 1.45 ± 0.11 | 1.49 ± 0.06 |
| 31 | α-humulene | 1673 | 1667 | RI; MS | 0.90 ± 0.02 | 0.86 ± 0.05 | 0.99 ± 0.03 |
| 32 | γ-muurolene | 1691 | 1690 | RI; MS | 0.24 ± 0.004 | 0.16 ± 0.01 | 0.32 ± 0.01 |
| 33 | γ-curcumene | 1693 | 1692 | RI; MS | 0.08 ± 0.01 | nd | nd |
| 34 | α-terpineol | 1700 | 1694 | RI; MS | 1.67 ± 0.04 | 0.64 ± 0.06 | 0.36 ± 0.01 |
| 35 | borneol | 1704 | 1700 | RI; MS | 0.50 ± 0.01 | 0.63 ± 0.06 | 7.71 ± 0.24 |
| 36 | germacrene D | 1713 | 1708 | RI; MS | nd | 0.28 ± 0.02 | nd |
| 37 | bicyclosesquiphellandrene | 1715 | 1706 | RI; MS | 0.12 ± 0.01 | 0.39 ± 0.03 | 0.99 ± 0.06 |
| 38 | β-selinene | 1724 | 1717 | RI; MS | 1.62 ± 0.05 | 1.29 ± 0.09 | 1.90 ± 0.12 |
| 39 | α-selinene | 1729 | 1725 | RI; MS | 1.75 ± 0.05 | 0.87 ± 0.08 | 1.07 ± 0.04 |
| 40 | β-curcumene | 1744 | 1737 | RI; MS | 0.26 ± 0.004 | nd | 0.50 ± 0.03 |
| 41 | δ-cadinene | 1761 | 1756 | RI; MS | 1.52 ± 0.05 | 3.06 ± 0.03 | 7.06 ± 0.38 |
| 42 | γ-cadinene | 1764 | 1763 | RI; MS | 0.52 ± 0.02 | nd | nd |
| 43 | citronellol | 1767 | 1764 | RI; MS | 0.09 ± 0.005 | nd | nd |
| 44 | cis-cadina-1(2),4-diene | 1784 | 1788 | RI; MS | 0.13 ± 0.005 | nd | 0.45 ± 0.03 |
| 45 | germacrene B | 1830 | 1824 | RI; MS | 0.13 ± 0.01 | nd | nd |
| 46 | cis-calamenene | 1839 | 1834 | RI; MS | 0.01 ± 0.00 | 0.07 ± 0.01 | 0.15 ± 0.01 |
| 47 | epicubebol | 1893 | 1900 | RI; MS | nd | 0.14 ± 0.03 | 1.00 ± 0.06 |
| 48 | α-calacorene | 1925 | 1921 | RI; MS | nd | nd | 0.11 ± 0.01 |
| 49 | cubebol | 1944 | 1942 | RI; MS | nd | 0.17 ± 0.02 | 1.70 ± 0.12 |
| 50 | caryophyllene oxide | 1991 | 1986 | RI; MS | nd | 0.12 ± 0.03 | 0.38 ± 0.12 |
| 51 | (E)-nerolidol | 2042 | 2036 | RI; MS | 1.52 ± 0.03 | 0.36 ± 0.06 | 1.38 ± 0.11 |
| 52 | humulene epoxide II | 2051 | 2047 | RI; MS | nd | nd | 0.22 ± 0.00 |
| 53 | cubenol | 2066 | 2068 | RI; MS | 0.17 ± 0.005 | 0.28 ± 0.04 | 1.10 ± 0.06 |
| 54 | 1,10-di-epi-cubenol | 2073 | 2074 | RI; MS | 0.23 ± 0.01 | 0.45 ± 0.09 | 1.71 ± 0.11 |
| 55 | globulol | 2082 | 2082 | RI; MS | 0.09 ± 0.00 | nd | nd |
| 56 | guaiol | 2094 | 2089 | RI; MS | 0.15 ± 0.004 | nd | nd |
| 57 | spathulenol | 2131 | 2127 | RI; MS | 0.09 ± 0.01 | 0.08 ± 0.02 | 1.05 ± 0.06 |
| 58 | τ-cadinol | 2177 | 2175 | RI; MS | 0.21 ± 0.01 | 0.12 ± 0.02 | 0.40 ± 0.02 |
| 59 | trans-muurolol | 2205 | 2209 | RI; MS | 0.21 ± 0.01 | 0.19 ± 0.04 | 0.73 ± 0.05 |
| 60 | farnesol isomer | 2356 | 2357 | RI; MS | 0.11 ± 0.02 | nd | nd |
| Compounds identified | 46 | 44 | 47 | ||||
| Total identified (%) | 95.66 | 97.35 | 92.72 | ||||
| Monoterpene hydrocarbons | 72.84 | 77.8 | 48.38 | ||||
| Oxygenated monoterpenes | 4.41 | 2.72 | 11.79 | ||||
| Sesquiterpene hydrocarbons | 15.49 | 14.34 | 22.29 | ||||
| Oxygenated sesquiterpenes | 2.78 | 1.91 | 9.67 | ||||
| Others | 0.14 | 0.57 | 0.6 | ||||
| DPPH | ABTS | FRAP | |
|---|---|---|---|
| μmol TE /g | μmol TE /g | μmol TE /g | |
| Leaf Oil | 0.92 ± 0.06 | 7.97 ± 0.41 | 30.89 ± 1.11 |
| Stem Oil | 1.52 ± 0.11 | 15.26 ± 0.72 | 34.77 ± 1.46 |
| Root Oil | 4.21 ± 0.05 | 25.52 ± 0.22 | 48.79 ± 1.08 |
| BHT | 2071.96 ± 15.54 | 5028.80 ± 41.66 | 2492.60 ± 25.33 |
| Species | Leaf Oil | Stem Oil | Root Oil | Gentamycin Sulfate | Amphotericin B | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | |
| S. aureus | 1.56 | 3.13 | 1.56 | 3.13 | 0.78 | 1.56 | 0.016 | 0.016 | / | / |
| S. lentus | 3.13 | 6.25 | 3.13 | 6.25 | 3.13 | 6.25 | 0.004 | 0.004 | / | / |
| B. atrophaeus | 50.0 | 100.0 | 100.0 | 100.0 | 25.0 | 50.0 | 0.0003 | 0.0003 | / | / |
| p. aeruginosa | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 12.5 | 0.002 | 0.002 | / | / |
| E. coli | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 12.5 | 0.016 | 0.016 | / | / |
| C. albicans | 3.13 | 6.25 | 3.13 | 6.25 | 3.13 | 6.25 | / | / | 0.0004 | 0.0008 |
| Concentratio (μg/mL) | Acetylcholinesterase Inhibition (%) | Butyrylcholinesterase Inhibition (%) | |
|---|---|---|---|
| Leaf Oil | 20 | 4.80 ± 0.23 | NA |
| 2 | NA | NA | |
| Stem Oil | 20 | 5.44 ± 0.11 | 1.68 ± 0.14 |
| 2 | NA | NA | |
| Root Oil | 20 | 0.83 ± 0.10 | NA |
| 2 | NA | NA | |
| Tacrine | 0.33 | 55.39 ± 2.79 | / |
| Iso-OMPA | 0.86 | / | 48.54 ± 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Ma, C.; Zhu, G. Chemical Composition and Biological Activities of Essential Oils from the Leaves, Stems, and Roots of Kadsura coccinea. Molecules 2021, 26, 6259. https://doi.org/10.3390/molecules26206259
Zhao T, Ma C, Zhu G. Chemical Composition and Biological Activities of Essential Oils from the Leaves, Stems, and Roots of Kadsura coccinea. Molecules. 2021; 26(20):6259. https://doi.org/10.3390/molecules26206259
Chicago/Turabian StyleZhao, Tianming, Chao Ma, and Guofei Zhu. 2021. "Chemical Composition and Biological Activities of Essential Oils from the Leaves, Stems, and Roots of Kadsura coccinea" Molecules 26, no. 20: 6259. https://doi.org/10.3390/molecules26206259