Inhibitory Role of Berberine, an Isoquinoline Alkaloid, on NLRP3 Inflammasome Activation for the Treatment of Inflammatory Diseases
Abstract
:1. Introduction
2. NLRP3 Inflammasome Activation and Its Role in Inflammatory Disorders
2.1. NLRP3 Inflammasome
2.2. Activation of NLRP3 Inflammasome via Host-Derived Activating Signals
2.3. NLRP3 Inflammasome Associated Human Disease
2.4. Role of the NLRP3 Inflammasome in Cancer
3. Berberine
3.1. Chemistry and Pharmacokinetics
3.2. Pharmacological Activities of BRB
- BRB has shown to exert diverse effects in intracellular oxidative stress. Many studies have demonstrated its antioxidant activity whereby it has been shown to induce the scavenging of ROS/RNS, chelation of metal ions, increase in antioxidant effects of endogenous substances, superoxide dismutase activity, and decrease in lipid peroxidation [39]. On the contrary, BRB has also been shown to increase ROS production, which subsequently activated several apoptotic signaling pathways such as JNK, ERK1/2, and MAPK, as well as Akt and Ca2+-dependent pathways [42].
- The nucleic acid affinity of BRB has been proposed to be partially responsible for its anticancer efficacy. BRB binds strongly with DNA with more affinity for polyadenylic acid [poly(A)] than other polynucleotides [48]. The binding of BRB with nuclear DNA has been shown to induce dsDNA breaks, leading to activation of the p53-dependent pathway, inducing cell cycle arrest and apoptosis [49]. Similarly, BRB binding to mRNA inhibits the process of translation and translocation [42].
- BRB also inhibits several carcinogenesis-related enzymes by directly inhibiting cytosolic arylamine N-acetyltransferase, telomerase, and topoisomerase activity, thus acting as a potent topoisomerase poison. BRB also suppresses the translational and transcriptional expression of cytosolic arylamine N-acetyltransferase and cyclooxygenase-2 [48,50].
- BRB induces both p53-dependent G1 arrest and p53-independent G2/M cell cycle arrest. BRB suppresses the MDM2 inhibitor protein at the post-transcriptional level, thus upregulating p53 expression, leading to G1 arrest and apoptosis. G1 arrest has also been associated with the BRB-induced overexpression of CIP1/p21 and Kip1/p27 proteins and downregulation of cyclin-dependent kinases (cdk2, cdk4, and cdk6), while BRB-induced G2/M arrest is associated with the downregulation of cyclin B1 and upregulation of Wee1, leading to apoptotic cell death [43]. Thus, BRB has the potential to induce tumor cell death irrespective of their p53 status [50].
- BRB has been shown to induce apoptotic cell death by affecting several apoptotic signaling pathways, including p53-dependent and -independent, caspase-dependent mitochondrial, Fas receptor-dependent, and caspase-independent pathways [42,50]. BRB has also been shown to induce apoptosis by targeting the redox/ROS and JNK/p38 signaling pathways [51], as well as two pro-apoptotic proteins: ATF3 mediated in a p53-dependent manner and NAG-1 involving several pathways, PKC, ERK, and GSK-3β [52].
- The anti-inflammatory activity of BRB is mediated by the inhibition of the pro-inflammatory NF-kB pathway, which is attributed to the inhibition of IκB kinase (IKK) activation, causing the stabilization of IκB alpha, leading to the dephosphorylation and nuclear translocation of p65, and finally inhibiting the reporter activity of NF-κB. The inhibition, in turn, resulted in the suppression of NF-κB-regulated gene products involved in anti-apoptosis (Bcl-XL, Survivin, IAP1, IAP2, and cFLIP), proliferation (cyclin D1), inflammation (COX-2), and invasion (MMP-9) [53].
- The anti-invasion or anti-metastatic potential of BRB in cancer is mainly ascribed to the downregulation of nuclear transcription factors: c-fos, c-jun, and NF-κB; inhibition of TNF-α-induced MMP-9 expression, suppression of MMP-1, -2, and -9 and u-PA, through MAPK and NF-αB signaling pathways; and inhibition of the RhoA signaling pathway [48,50].
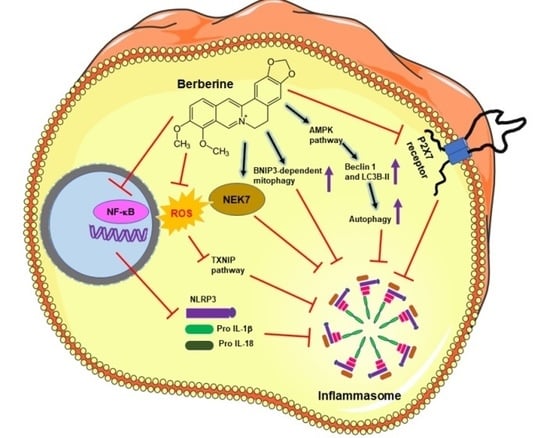
3.3. Inhibitory Action of Berberine on Inflammasome Pathway and Associated Diseases
3.3.1. Cancer
3.3.2. Liver Disease
3.3.3. Macrophages and Macrophages-Mediated Diseases
3.3.4. Neurological Disorders
3.3.5. Nephropathy
3.3.6. Arthritis
3.3.7. Viral Infection
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Bachmann, M.C.; Bellalta, S.; Basoalto, R.; Gómez-Valenzuela, F.; Jalil, Y.; Lépez, M.; Matamoros, A.; Von Bernhardi, R. The Challenge by Multiple Environmental and Biological Factors Induce Inflammation in Aging: Their Role in the Promotion of Chronic Disease. Front. Immunol. 2020, 11, 570083. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R.A. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Menu, P.; Vince, J.E. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.P.; Leal, V.N.C.; De Lima, D.S.; Reis, E.C.; Pontillo, A. Inflammasome genetics and complex diseases: A comprehensive review. Eur. J. Hum. Genet. 2020, 28, 1307–1321. [Google Scholar] [CrossRef]
- Bagherniya, M.; Khedmatgozar, H.; Fakheran, O.; Xu, S.; Johnston, T.P.; Sahebkar, A. Medicinal plants and bioactive natural products as inhibitors of NLRP3 inflammasome. Phytother. Res. 2021, 35, 4804–4833. [Google Scholar] [CrossRef]
- Olcum, M.; Tastan, B.; Ercan, I.; Eltutan, I.B.; Genc, S. Inhibitory effects of phytochemicals on NLRP3 inflammasome activation: A review. Phytomedicine 2020, 75, 153238. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.-Y.; Li, B.; Zhu, W.-L.; Shi, J.-Y.; Jia, Q.; Li, Y.-M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2016, 38, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Georgin-Lavialle, S.; Grateau, G.; Amselem, S.; Giurgea, I.; Karabina, S.-A. Inflammasome biology, molecular pathology and therapeutic implications. Pharmacol. Ther. 2018, 187, 133–149. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Christgen, S.; Place, D.E.; Kanneganti, T.-D. Toward targeting inflammasomes: Insights into their regulation and activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Rathinam, V.A.K.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Dong-Min, S.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar]
- Zamani, P.; Oskuee, R.K.; Atkin, S.L.; Navashenaq, J.G.; Sahebkar, A. MicroRNAs as important regulators of the NLRP3 inflammasome. Prog. Biophys. Mol. Biol. 2020, 150, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the role of NLRP3 inflammasome in diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Wilson, S.P.; Cassel, S.L. Inflammasome-Mediated Autoinflammatory Disorders. Postgrad. Med. 2010, 122, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front. Immunol. 2020, 11, 1444. [Google Scholar] [CrossRef]
- Jang, J.-H.; Kim, D.-H.; Surh, Y.-J. Dynamic roles of inflammasomes in inflammatory tumor microenvironment. npj Precis. Oncol. 2021, 5, 18. [Google Scholar] [CrossRef]
- He, Q.; Fu, Y.; Tian, D.; Yan, W. The contrasting roles of inflammasomes in cancer. Am. J. Cancer Res. 2018, 8, 566–583. [Google Scholar]
- Kantono, M.; Guo, B. Inflammasomes and Cancer: The Dynamic Role of the Inflammasome in Tumor Development. Front. Immunol. 2017, 8, 1132. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Bhol, C.S.; Praharaj, P.P.; Panigrahi, D.P.; Patra, S.; Singh, A.; Patil, S.; Dhiman, R.; et al. Inflammasomes in cancer: Effect of epigenetic and autophagic modulations. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Chung, C.; Seo, W.; Silwal, P.; Jo, E.-K. Crosstalks between inflammasome and autophagy in cancer. J. Hematol. Oncol. 2020, 13, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Alehashemi, S.; Goldbach-Mansky, R. Human Autoinflammatory Diseases Mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-Inflammasome Dysregulation Updates on Diagnosis, Treatment, and the Respective Roles of IL-1 and IL-18. Front. Immunol. 2020, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Vong, C.T.; Tseng, H.H.L.; Yao, P.; Yu, H.; Wang, S.; Zhong, Z.; Wang, Y. Specific NLRP3 inflammasome inhibitors: Promising therapeutic agents for inflammatory diseases. Drug Discov. Today 2021, 26, 1394–1408. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.; Li, B.; Kombe, A.J.K.; Jin, T.; Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Li, X.; Liu, Y.; Xia, Y.; Chang, R.; Zhang, C. Inflammasome inhibitors: Promising therapeutic approaches against cancer. J. Hematol. Oncol. 2019, 12, 755. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar]
- Singh, B.; Katare, A.K. Botanical Sources, Chemistry Aspects and Biological Functions of Berberine: An Updated Critical Review. In Botanical Leads for Drug Discovery; Singh, B., Ed.; Springer: Singapore, 2020; pp. 421–462. [Google Scholar]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.K.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Central Nerv. Syst. Agents Med. Chem. 2019, 19, 154–170. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 2009, 24, 317–324. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Rahmat, A.; Ismail, P.; Ling, K.-H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of berberine and its derivatives on cancer: A systems pharmacology review. Front. Pharmacol. 2020, 10, 1461. [Google Scholar] [CrossRef] [Green Version]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.; Šmejkal, K.; Malaník, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The anti-cancer mechanisms of berberine: A review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, D.; Liu, L.; Wu, Z.; Cao, M. Combating Neurodegenerative diseases with the plant alkaloid berberine: Molecular mechanisms and therapeutic potential. Curr. Neuropharmacol. 2019, 17, 563–579. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat. Res. Mol. Mech. Mutagen. 2009, 662, 75–83. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-H.; Hsieh, Y.-S.; Kuo, H.-C.; Teng, C.-Y.; Huang, H.-I.; Wang, C.-J.; Yang, S.-F.; Liou, Y.-S.; Kuo, W.-H. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch. Toxicol. 2007, 81, 719–728. [Google Scholar] [CrossRef]
- Piyanuch, R.; Sukhthankar, M.; Wandee, G.; Baek, S.J. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007, 258, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.K.; Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Berberine modifies cysteine 179 of IκBα kinase, suppresses nuclear factor-κB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008, 68, 5370–5379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Fan, X.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.; Liu, J. Berberine inhibits NLRP3 inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern Med. 2019, 19, 216. [Google Scholar] [CrossRef] [Green Version]
- Mai, W.; Xu, Y.; Xu, J.; Zhao, D.; Ye, L.; Yu, G.; Wang, Z.; Lu, Q.; Lin, J.; Yang, T.; et al. Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP axis. Front. Pharmacol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivoli, E.; Cappon, A.; Milani, S.; Piombanti, B.; Provenzano, A.; Novo, E.; Masi, A.; Navari, N.; Narducci, R.; Mannaioni, G.; et al. NLRP3 inflammasome as a target of berberine in experimental murine liver injury: Interference with P2X7 signalling. Clin. Sci. 2016, 130, 1793–1806. [Google Scholar] [CrossRef]
- Li, C.G.; Yan, L.; Jing, Y.Y.; Xu, L.H.; Liang, Y.D.; Wei, H.X.; Hu, B.; Pan, H.; Zha, Q.B.; Ouyang, D.Y.; et al. Berberine augments ATP-induced inflammasome activation in macrophages by enhancing AMPK signaling. Oncotarget 2017, 8, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Huang, K.; Lin, X.; Chen, Q.; Lin, S.; Feng, X.; Zhen, C.; Huang, M.; Wang, S. Berberine attenuates NLRP3 inflammasome activation in macrophages to reduce the secretion of Interleukin-1β. Ann. Clin. Lab. Sci. 2017, 47, 720–728. [Google Scholar]
- Huang, Z.; Ye, B.; Han, J.; Kong, F.; Shan, P.; Lu, Z.; Huang, Z.; Huang, W. NACHT, LRR and PYD domains-containing protein 3 inflammasome is activated and inhibited by berberine via toll-like receptor 4/myeloid differentiation primary response gene 88/nuclear factor-κB pathway, in phorbol 12-myristate 13-acetate-induced macrophages. Mol. Med. Rep. 2018, 17, 2673–2680. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Feng, L.; Xu, F.; Sun, Y.; Ma, Y.; Zhang, X.; Liu, H.; Xu, G.; Wu, X.; Shen, Y.; et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking berberine to insulin resistance improvement. Biomed. Pharmacother. 2017, 89, 864–874. [Google Scholar] [CrossRef]
- Huang, S.; Liu, H.; Lin, Y.; Liu, M.; Li, Y.; Mao, H.; Zhang, Z.; Zhang, Y.; Ye, P.; Ding, L.; et al. Berberine protects against NLRP3 inflammasome via ameliorating autophagic impairment in MPTP-induced Parkinson’s disease model. Front. Pharmacol. 2021, 11, 618787. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Liu, D.; Zheng, J.; Xie, J.; Chen, J.; Zeng, H.; Su, Z.; Li, Y. Effect of berberine on hyperuricemia and kidney injury: A network pharmacology analysis and experimental validation in a mouse model. Drug Des. Dev. Ther. 2021, 15, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Wen, C.Y.Z.; Chen, Z.; Wang, Y.; Huang, Y.; Tu, S.H. Effects of berberine on NLRP3 and IL-1 β mxpressions in Monocytic THP-1 cells with monosodium urate crystals-induced inflammation. Biomed. Res. Int. 2016, 2016, 2503703. [Google Scholar] [PubMed]
- Dinesh, P.; Rasool, M.K. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int. Immunopharmacol. 2017, 44, 26–37. [Google Scholar] [CrossRef]
- Liu, H.; You, L.; Wu, J.; Zhao, M.; Guo, R.; Zhang, H.; Su, R.; Mao, Q.; Deng, D.; Hao, Y. Berberine suppresses influenza virus-triggered NLRP3 inflammasome activation in macrophages by inducing mitophagy and decreasing mitochondrial ROS. J. Leukoc. Biol. 2020, 108, 253–266. [Google Scholar] [CrossRef]
- Zeng, Q.; Deng, H.; Li, Y.; Fan, T.; Liu, Y.; Tang, S.; Wei, W.; Liu, X.; Guo, X. Berberine directly targets the NEK7 protein to block the NEK7-NLRP3 interaction and exert anti-inflammatory activity. J. Med. Chem. 2021, 64, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2015, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Croasdell, A.; Sime, P.J.; Phipps, R.P. Resolvin D2 decreases TLR4 expression to mediate resolution in human monocytes. FASEB J. 2016, 30, 3181–3193. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.; Phillips, O.; Fukumoto, J.; Fukumoto, I.; Parthasarathy, P.T.; Mandry, M.; Cho, Y.; Lockey, R.F.; Kolliputi, N. Resolvins Decrease Oxidative Stress Mediated Macrophage and Epithelial Cell Interaction through Decreased Cytokine Secretion. PLoS ONE 2015, 10, e0136755. [Google Scholar] [CrossRef] [Green Version]
- Bohr, S.; Patel, S.J.; Sarin, D.; Irimia, D.; Yarmush, M.L.; Berthiaume, F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2012, 21, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Kain, V.; Ingle, K.A.; Colas, R.A.; Dalli, J.; Prabhu, S.D.; Serhan, C.N.; Joshi, M.; Halade, G.V. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell Cardiol. 2015, 84, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clària, J.; Dalli, J.; Yacoubian, S.; Gao, F.; Serhan, C.N. Resolvin D1 and Resolvin D2 Govern Local Inflammatory Tone in Obese Fat. J. Immunol. 2012, 189, 2597–2605. [Google Scholar] [CrossRef]
- Rius, B.; Titos, E.; Morán-Salvador, E.; López-Vicario, C.; García-Alonso, V.; González-Périz, A.; Arroyo, V.; Claria, J. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 2013, 28, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Lopategi, A.; Flores-Costa, R.; Rius, B.; López-Vicario, C.; Alcaraz-Quiles, J.; Titos, E.; Clària, J. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J. Leukoc. Biol. 2018, 105, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Chen, Z.; Bhat, O.M.; Zhang, Q.; Abais-Battad, J.M.; Conley, S.M.; Ritter, J.K.; Li, P.-L. NLRP3 inflammasome as a novel target for docosahexaenoic acid metabolites to abrogate glomerular injury. J. Lipid Res. 2017, 58, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Nakahira, K.; Dalli, J.; Siempos, I.I.; Norris, P.C.; Colas, R.A.; Moon, J.S.; Shinohara, M.; Hisata, S.; Howrylak, J.A.; et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am. J. Respir Crit Care Med. 2017, 196, 713–726. [Google Scholar] [CrossRef]



| Disease | Activators/Stimulator |
|---|---|
| Obesity | Saturated free fatty acids, cholesterol crystals, ceramides, adipokines, and hyperglycemia |
| Type 2 Diabetes | Saturated free fatty acids, ceramides, high levels of glucose, uric acid, and Islet amyloid polypeptide |
| Atherosclerosis | Cholesterol crystals, calcium phosphate crystals, and oxidized LDL |
| Cardiovascular diseases ypertension, ischemic injury, cardiomyopathy, and myocardial infarction) | Low-density lipoprotein (LDL), cholesterol crystal, external irritants, and HIV-1 infection |
| Liver Disease (Alcoholic and Nonalcoholic steatohepatitis and Viral hepatitis) | Cholesterol crystals, ethanol, nanoparticles (rare-earth oxide (REO), quantum dots, and mesoporous silica), and hepatitis C virus (HCV) infection |
| Parkinson’s Disease | Aggregated α-synuclein |
| Alzheimer’s Disease | Amyloid-β plaques, Tau monomers, and oligomers |
| Inhibitor Class | Examples | Potential Mechanism(s) of Inhibition |
|---|---|---|
| Direct Inhibitors | ||
| Small Molecules | MCC950 | Binds and inhibits the ATPase activity NACHT domain, thus hindering NLRP3 oligomerization. |
| 3,4-Methylenedioxy-β-nitrostyrene (MNS) | Binds to the NACHT and LRR domains, thus inhibiting ATPase activity and NLRP3 oligomerization. | |
| CY-09 | Binds and inhibits ATPase activity of NACHT domain and hinders NLRP3 oligomerization. | |
| (N-[3′,4′-dimethoxycinnamoyl]-anthranilic acid (Tranilast) | Binds to NACHT domain and blocks NLRP3 oligomerization. | |
| OLT1177 | Impedes ATPase activity and obstructs NLRP3 oligomerization. | |
| Oridonin | Binds cysteine 279 of NLRP3 NACHT domain via a covalent bond, blocks NLRP3-NEK7 interaction, and hinders subsequent NLRP3 inflammasome activation. | |
| Indirect Inhibitors | ||
| Small Molecules | Glyburide | Inhibits ATP-sensitive K+ channels and blocks ASC aggregations. |
| 16673-34-0 | Interferes with the NLRP3 protein activation and/or the aggregate with the ASC scaffold, and blocks inflammasome activation. | |
| JC124 | Downregulates NLRP3, ASC, caspase-1, pro-IL-1β, TNF-α expression; and inhibits inducible nitric oxide synthase (iNOS). | |
| FC11A-2 | Hinders autocleavage of procaspase-1 and diminishes activated caspase-1 level, and suppresses IL-1β/18 release. | |
| Parthenolide | Induces cysteine modifications and inhibits caspase-1 activation and ATPase activity of NLRP3 protein. | |
| VX-740 and VX-765 | Blocks caspase-1 activation by covalent modification of the active site catalytic cysteine and resultant cleavage of pro-IL-1β/18. | |
| Bay 11-7082 | Modifies cysteine residues of ATPase site of NLRP3, and inhibits APTase activity of NLRP3 and ASC organization. | |
| β-Hydroxybutyrate (BHB) | Inhibits K+ efflux, inhibits ASC oligomerization, and suppresses IL-1ß and IL-18 production. | |
| Interferons | IFN-α and IFN-β | Phosphorylates STAT1 transcription factor, suppresses NLRP3 inflammasomes, and inhibits caspase-1-dependent IL-1β activation. Induces STAT-dependent IL-10 production and reduces pro-IL-1α and pro-IL-1β levels. |
| Autophagy Inducers | Resveratrol | Induces autophagy, suppresses mitochondrial damage, and inhibits NLRP3 inflammasome activation. |
| Arglabin | Induces autophagy, reduces inflammation and cholesterol levels, and inhibits NLRP3 inflammasome activation. | |
| Cannabinoid receptor 2 (CB2R) | Induces autophagy and inhibits NLRP3 inflammasome priming and activation. | |
| MicroRNAs | MicroRNA-223 | Binds to the 3′ UTR of the NLRP3 transcript, suppresses NLRP3 protein expression, and prevents priming of NLRP3 inflammasome and IL-1β secretion. |
| Family | Plant Species | Used Parts |
|---|---|---|
 Annonaceae | nnickia polycarpa | Bark |
| nickia chlorantha | Bark | |
| Annickiapilosa | Bark | |
| Rollinia mucosa | Fruits | |
 Berberidaceae | Berberis vulgaris | Stem, root |
| Berberis aristate | Bark, root, stem, fruits, extract | |
| Berberis petiolaris | Root | |
| Thunbergia | Stem | |
| Aquifolium | Root | |
| Asiatica | Bark, root, stem | |
 Ranunculaceae | Coptis Chinensis | Root |
| Coptis teeta | Rhizome | |
| Coptis japonica | Rhizome | |
| Hydrastis canadensis | Whole plant | |
| Xanthorhiza simplicissima | Roots, stem, leaves | |
 Menispermaceae | Tinospora sinensis | Stem |
| Tinospora cordifolia | Stem | |
 Rutaceae | Zanthoxylum schreberi | Stem, branches |
| Zanthoxylum armatum | Stem | |
| Phellodendron lavallei | Bark | |
| Phellodendron amurense | Barks, branches, leaves | |
| Evodia meliaefolia | Bark | |
 Papaveraceae | Argemone albiflora | Aerial parts, roots |
| Argemone mexicana | Epigeal parts, leaves, seeds, roots, fruit capsules, latex | |
| Argemone ochroleuca | Seeds | |
| Argemone squarrosa | Aerial part |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbadhikary, P.; George, B.P.; Abrahamse, H. Inhibitory Role of Berberine, an Isoquinoline Alkaloid, on NLRP3 Inflammasome Activation for the Treatment of Inflammatory Diseases. Molecules 2021, 26, 6238. https://doi.org/10.3390/molecules26206238
Sarbadhikary P, George BP, Abrahamse H. Inhibitory Role of Berberine, an Isoquinoline Alkaloid, on NLRP3 Inflammasome Activation for the Treatment of Inflammatory Diseases. Molecules. 2021; 26(20):6238. https://doi.org/10.3390/molecules26206238
Chicago/Turabian StyleSarbadhikary, Paromita, Blassan P. George, and Heidi Abrahamse. 2021. "Inhibitory Role of Berberine, an Isoquinoline Alkaloid, on NLRP3 Inflammasome Activation for the Treatment of Inflammatory Diseases" Molecules 26, no. 20: 6238. https://doi.org/10.3390/molecules26206238