The Influence of the Preparation Method on the Physico-Chemical Properties and Catalytic Activities of Ce-Modified LDH Structures Used as Catalysts in Condensation Reactions
Abstract
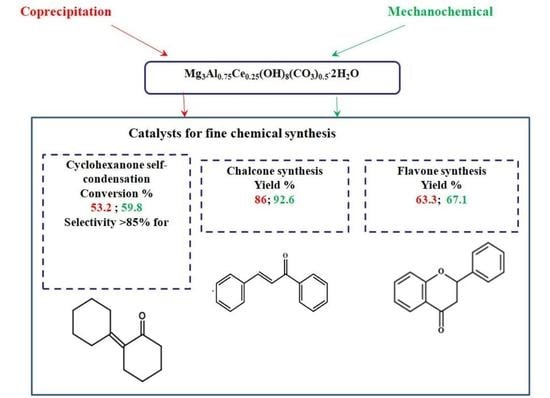
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. SEM Analysis
2.3. Chemical Composition
2.4. DRIFT Measurements
2.5. Raman Analysis
2.6. DRUV-VIS NIR Analysis
2.7. Determination of Acido-Bascity and Textural Properties
2.8. Catalytic Activity Tests
2.8.1. Self-Condensation
2.8.2. Claisen-Schmidt Condensation
3. Materials and Methods
3.1. Synthesis of The LDHs and Their Corresponding Mixed Oxides
3.2. Characterization Techniques
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tongamp, W.; Zhang, Q.; Saito, F. Mechanochemical route for synthesizing nitrate form of layered double hydroxide. Powder Technol. 2008, 185, 43–48. [Google Scholar] [CrossRef]
- Mobley, J.K.; Crocker, M. Catalytic oxidation of alcohols to carbonyl compounds over hydrotalcite and hydrotalcite supported catalysts. RSC Adv. 2015, 81, 65780–65797. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.; Hnefeld, U. Introduction: Green Chemistry and Catalysis. In Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007; pp. 1–14. [Google Scholar] [CrossRef]
- Yu, H.; Xu, B.; Bian, L.; Gao, H. Influence on Structure of Layered Double Hydroxides with Different Methods Synthesis. Adv. Mater. Res. 2011, 160, 656–660. [Google Scholar] [CrossRef]
- Baskaran, T.; Christopher, J.; Sakthivel, A. Progress on layered hydrotalcite (H.T.) materials as potential support and catalytic materials. RSC Adv. 2015, 120, 98853–98875. [Google Scholar] [CrossRef]
- Tongamp, W.; Zhang, Q.; Saito, F. Preparation of meixnerite (Mg-Al-OH) type layered double hydroxide by a mechanochemical route. J. Mater. Sci. 2007, 42, 9210–9215. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Hu, H.; Zhang, Q.; Tao, D. Mechanochemical synthesis of novel Pt modified ZnAl-LDH for effective ciprofloxacin photodegradation. J. Solid State Chem. 2020, 290, 121594. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, W. Mechano-hydrothermal preparation of Li-Al-OH layered double hydroxides. Sol. State Sci. 2018, 79, 93–98. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Su, L.; Chen, L.; Wu, J.; Meng, J. Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride). Materials 2020, 13, 5223. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Pavel, O.D.; Zăvoianu, R.; Bîrjega, R.; Angelescu, E.; Pârvulescu, V.I. Mechanochemical versus co-precipitated synthesized lanthanum-doped layered materials for olefin oxidation. Appl. Catal. A Gen. 2017, 542, 10–20. [Google Scholar] [CrossRef]
- Pavel, O.D.; Stamate, A.-E.; Zăvoianu, R.; Bucur, I.C.; Bîrjega, R.; Angelescu, E.; Parvulescu, V.I. Mechano-chemical versus coprecipitation for the preparation of Y-modified LDHs for cyclohexene oxidation and Claisen-Schmidt condensations. Appl. Catal. A Gen. 2020, 605, 117797. [Google Scholar] [CrossRef]
- Lorenzo, D.; Santos, A.; Simón, E.; Romero, A. Kinetic of Alkali Catalyzed Self-Condensation of Cyclohexanone. Ind. Eng. Chem. Res. 2013, 52, 2257–2265. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kadlec, D.; Velvarská, R.; Kubička, D. Using Mg-Al Mixed Oxide and Reconstructed Hydrotalcite as Basic Catalysts for Aldol Condensation of Furfural and Cyclohexanone. ChemCatChem 2018, 10, 1464–1475. [Google Scholar] [CrossRef]
- Wan, M.; Liang, D.; Wang, L.; Zhang, X.; Yang, D.; Li, G. Cycloketone condensation catalyzed by zirconia: Origin of reactant selectivity. J. Catal. 2018, 361, 186–192. [Google Scholar] [CrossRef]
- Deng, Q.; Nie, G.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Highly selective self-condensation of cyclic ketones using MOF-encapsulating phosphotungstic acid for renewable high-density fuel. Green Chem. 2015, 17, 4473–4481. [Google Scholar] [CrossRef]
- Kang, L.; Wei, B. Synthesis of α,α′-bisbenzylidenecycloalkanones catalyzed by amberlyst 15. Chemistry Bulletin/Huaxue Tongbao 2007, 70, 801–804. [Google Scholar]
- Hazarkhani, H.; Kumar, P.; Kondiram, K.S.; Gadwal, I.M.S. Highly Selective Claisen–Schmidt Condensation Catalyzed by Silica Chloride Under Solvent-Free Reaction Conditions. Synth. Commun. 2010, 40, 2887–2896. [Google Scholar] [CrossRef]
- Gu, Y.; Gao, Q.; Suo, J. Self-condensation of cyclohexanone over keggin type lanthanum phosphotungstate catalyst. Shiyou Huagong/Petrochem. Technol. 2009, 38, 759–762. [Google Scholar]
- Mahajan, Y.S.; Kamath, R.S.; Kumbhar, P.S.; Mahajani, S.M. Self-condensation of cyclohexanone over ion exchange resin catalysts: Kinetics and selectivity aspects. Ind. Eng. Chem. 2008, 47, 25–33. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer Chalcones: Structural and Molecular Target Perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K. Therapeutic potential of chalcones as cardiovascular agents. Life Sci. 2016, 148, 154–172. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Crivoi, D.G.; Medina, F.; Tichit, D. Synthesis of Chalcone Using LDH/Graphene Nanocatalysts of Different Compositions. ChemEngineering 2019, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Yadav, G.D.; Wagh, D.P. Claisen-Schmidt Condensation using Green Catalytic Processes: A Critical Review. ChemistrySelect 2020, 5, 9059–9085. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Velty, A. Activated hydrotalcites as catalysts for the synthesis of chalcones of pharmaceutical interest. J. Catal. 2004, 221, 474–482. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.; Cen, K. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef]
- Ma, W.; Mashimo, T.; Tamura, S.; Tokuda, M.; Yoda, S.; Tsushida, M.; Koinuma, M.; Kubota, A.; Isobe, H.; Yoshiasa, A. Cerium oxide (CeO2-x) nanoparticles with high Ce3+ proportion synthesized by pulsed plasma in liquid. Ceram. Int. 2020, 46, 26502–26510. [Google Scholar] [CrossRef]
- Palmer, S.J.; Nguyen, T.; Frost, R.L. Synthesis and Raman spectroscopic characterisation of hydrotalcite with CO32− and VO3− anions in the interlayer. J. Raman Spectrosc. 2007, 38, 1602–1608. [Google Scholar] [CrossRef] [Green Version]
- Dronova, M.; Lair, V.; Vermaut, P.; Ringuedé, A.; An, V. Study of ceria thin films prepared via electrochemical deposition: Role of selected electrochemical parameters on growth kinetics. Thin Solid Films 2020, 693, 137674. [Google Scholar] [CrossRef]
- Palmer, S.J.; Frost, R.L.; Spratt, H.J. Synthesis and Raman spectroscopic study of Mg/Al,Fe hydrotalcites with variable cationic ratios. J. Raman Spectrosc. 2009, 40, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Guo, M.; Bandyopadhyay, P.; Lan, Q.; Xie, H.; Liu, G.; Liu, X.; Cheng, X.; Kim, N.H.; Lee, J.H. Two-dimensional materials modified layered double hydroxides: A series of fillers for improving gas barrier and permselectivity of poly(vinyl alcohol). Compos. B Eng. 2021, 207, 108568. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Fedel, M. Effect of Synthesis Conditions on the Controlled Growth of MgAl–LDH Corrosion Resistance Film: Structure and Corrosion Resistance Properties. Coatings 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Saedy, S. Synthesis and physicochemical characterizations of nanostructured Pt/Al2O3–CeO2 catalysts for total oxidation of VOCs. J. Hazard. Mater. 2011, 186, 1445–1454. [Google Scholar] [CrossRef]
- Bensalem, A.; Bozon-Verduraz, F.; Delamar, M.; Bugli, G. Preparation and Characterization of Highly Dispersed Silica-Supported Ceria. Appl. Catal. A 1995, 121, 81–93. [Google Scholar] [CrossRef]
- Rao, R.G.; Sahu, R.H. XRD and UV-Vis diffuse reflectance analysis of CeO2–ZrO2 solid solutions synthesized by combustion method. Proc. Indian Acad. Sci. 2001, 113, 651–658. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Eimer, G.A.; Casuscelli, S.G.; Chanquia, C.M.; Elías, V.; Crivello, M.E.; Herrero, E.R. The influence of Ti-loading on the acid behavior and on the catalytic efficiency of mesoporous Ti-MCM-41 molecular sieves. Catal. Today 2008, 133–135, 639–646. [Google Scholar] [CrossRef]
- Song, H.; Wang, J.; Wang, Z.; Song, H.; Li, F.; Jin, Z. Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst. J. Catal. 2014, 311, 257–265. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]








| Samples | Crystalline Phases | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LDH | CeO 2 | Ce2(CO3)2O·H2O | |||||||
| a (Å) | c (Å) | D003(nm) | D110(nm) | a (Å) | D111(nm) | a (Å) | b (Å) | c (Å) | |
| ICDD-references | Mg6Al2(CO3)(OH)16·4H2O (ICCD card 054-1030) | CeO2-cerianite (ICDD card 034-0394) | Ce2(CO3)2O·H2O (ICDD card 044-0617) | ||||||
| 3.052 | 22.446 | 5.411 | - | 7.322 | 8.568 | 5.019 | |||
| HTCe-PP | 3.066 | 23.756 | 6.3 | 11.7 | 5.415 | 4.6 | 7.358 | 8.576 | 5.030 |
| HTCe-MC | 3.063 | 23.525 | 8.0 | 8.9 | 5.417 | 3.4 | - | - | - |
| Samples | Crystalline Phases | |||
|---|---|---|---|---|
| Mixed Oxide Mg(Al)O | CeO2 | |||
| a (Å) | D200(nm) | a (Å) | D111(nm) | |
| ICDD references | MgO-periclase ICDD card 045-0946 | CeO2-cerianite ICDD card 034-0394 | ||
| 4.2112 | - | 5.411 | - | |
| Ce-PP | 4.187 | 3.8 | 5.386 | 3.9 |
| Ce-MC | 4.171 | 3.6 | 5.365 | 3.4 |
| Samples | Chemical Compositions (Atomic Ratios) | ||||
|---|---|---|---|---|---|
| EDS | XPS | ||||
| Mg/Al | Ce/Al | Mg/Al | Ce/Al | Ce3+/Ce4+ | |
| HTCe-PP | 3.09 | 0.11 | 2.07 | 0.25 | 0.98 |
| HTCe-MC | 3.24 | 0.29 | 2.85 | 0.13 | 1.07 |
| Ce-PP | 3.17 | 0.20 | 1.63 | 0.22 | 1.11 |
| Ce-MC | 3.03 | 0.31 | 2.19 | 0.19 | 0.80 |
| Solid | Surface Area (m2·g−1) | Total Acid Sites (mmol pyridine/g) | HB 1 (%) | Lewis Acid Sites (%) | Total Base Sites (mmol acrylic acid/g) | Strong Base Sites (mmol phenol/g) | Weak and Medium Strength (mmol/g) | Base/Acid Sites Ratio | Density (kg/m3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Real | Bulk | |||||||||
| Ce-MC | 200 | 0.376 | 4.4 | 95.6 | 3.98 | 2.62 | 1.36 | 10.59 | 2224.7 | 539.0 |
| Ce-PP | 184 | 0.611 | 76 | 24 | 2.0 | 1.98 | 0.02 | 3.27 | 4632.4 | 981.6 |
| Entry | Catalysts | Conv. of Cyclohexanone (%) | Sel. in (A) (%) | Sel. in (A1) (%) | Sel. in (B) (%) | Sel. in (B1) (%) |
|---|---|---|---|---|---|---|
| 1 | HTCe-PP | 33.5 | 76.5 | 11.1 | 8.3 | 4.1 |
| 2 | HTCe-MC | 35.1 | 84.3 | 13.4 | 1.9 | 0.4 |
| 3 | Ce-PP | 53.2 | 86.3 | 13.7 | 0.0 | 0.0 |
| 4 | Ce-MC | 59.8 | 87.9 | 12.1 | 0.0 | 0.0 |
| 5 | Ce-MC/recycled | 54.2 | 83.4 | 11.8 | 2.4 | 2.4 |
| 6 | Ce-MC/solvent | 34.1 | 87.5 | 11.1 | 1.0 | 0.3 |
| BA/ACP and BA/HAP Molar Ratios | Conv. of ACP (%) | Sel. to CH (%) | Yield of CH (%) | Conv. of HAP (%) | Sel. to FL (%) | Yield of FL (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce-PP | Ce-MC | Ce-PP | Ce-MC | Ce-PP | Ce-MC | Ce-PP | Ce-MC | Ce-PP | Ce-MC | Ce-PP | Ce-MC | |
| 1/1 | 37.1 | 42.3 | 100 | 100 | 37.1 | 42.3 | 57.2 | 79.1 | 77.7 | 78.8 | 44.4 | 62.3 |
| 5/1 | 77 | 78.2 | 94.5 | 96.2 | 72.8 | 75.2 | 84.3 | 85.2 | 70.5 | 75.9 | 59.4 | 64.7 |
| 10/1 | 93 | 97.1 | 92.5 | 95.4 | 86.0 | 92.6 | 94.1 | 96.2 | 67.3 | 69.7 | 63.3 | 67.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamate, A.-E.; Zăvoianu, R.; Pavel, O.D.; Birjega, R.; Matei, A.; Dumitru, M.; Brezeștean, I.; Osiac, M.; Marcu, I.-C. The Influence of the Preparation Method on the Physico-Chemical Properties and Catalytic Activities of Ce-Modified LDH Structures Used as Catalysts in Condensation Reactions. Molecules 2021, 26, 6191. https://doi.org/10.3390/molecules26206191
Stamate A-E, Zăvoianu R, Pavel OD, Birjega R, Matei A, Dumitru M, Brezeștean I, Osiac M, Marcu I-C. The Influence of the Preparation Method on the Physico-Chemical Properties and Catalytic Activities of Ce-Modified LDH Structures Used as Catalysts in Condensation Reactions. Molecules. 2021; 26(20):6191. https://doi.org/10.3390/molecules26206191
Chicago/Turabian StyleStamate, Alexandra-Elisabeta, Rodica Zăvoianu, Octavian Dumitru Pavel, Ruxandra Birjega, Andreea Matei, Marius Dumitru, Ioana Brezeștean, Mariana Osiac, and Ioan-Cezar Marcu. 2021. "The Influence of the Preparation Method on the Physico-Chemical Properties and Catalytic Activities of Ce-Modified LDH Structures Used as Catalysts in Condensation Reactions" Molecules 26, no. 20: 6191. https://doi.org/10.3390/molecules26206191