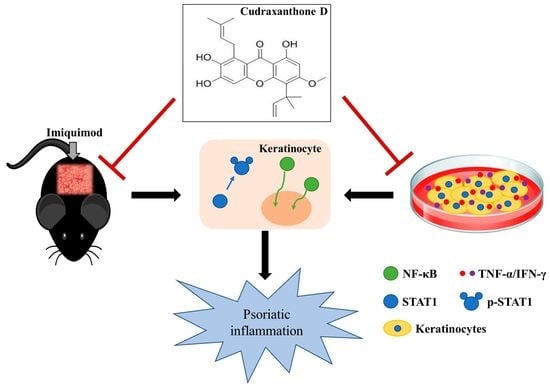
Cudraxanthone D Ameliorates Psoriasis-like Skin Inflammation in an Imiquimod-Induced Mouse Model via Inhibiting the Inflammatory Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. CD Reduced the Symptoms of Psoriasis-like Skin Inflammation
2.2. CD Reduced the Mediators of Psoriasis-Related Inflammation in an IMQ-Induced Mouse Model
2.3. CD Reduced the Inflammation-Related Gene Expression by Inhibiting NF-κB and STAT1 in Activated Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Reagents, Cell Maintenance, and Cell Viability
4.2. Plant Materials and Isolation of CD
4.3. Ethics Statement
4.4. Observation in the Back Skin of Mouse and Sample Collection
4.5. ELISA
4.6. qPCR
4.7. Histological and Immunohistochemistry Analysis
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lowes, M.A.; Bowcock, A.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na Takuathung, M.; Wongnoppavich, A.; Panthong, A.; Khonsung, P.; Chiranthanut, N.; Soonthornchareonnon, N.; Sireeratawong, S. Antipsoriatic Effects of Wannachawee Recipe on Imiquimod-Induced Psoriasis-Like Dermatitis in BALB/c Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 7931031. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, C.; Madonna, S.; Gisondi, P.; Girolomoni, G. The Interplay Between Keratinocytes and Immune Cells in the Pathogenesis of Psoriasis. Front. Immunol. 2018, 9, 1549. [Google Scholar] [CrossRef] [Green Version]
- Coondoo, A. The role of cytokines in the pathomechanism of cutaneous disorders. Indian J. Dermatol. 2012, 57, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.; Gelfand, J.; Gordon, K.B.; Gottlieb, A.B.; Koo, J.Y.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J. Am. Acad. Dermatol. 2009, 61, 451–485. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C.J.J. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Mason, A.; Mason, J.; Cork, M.; Hancock, H.; Dooley, G. Topical treatments for chronic plaque psoriasis of the scalp: A systematic review. Br. J. Dermatol. 2013, 169, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef]
- Wong, I.T.Y.; Shojania, K.; Dutz, J.; Tsao, N.W. Clinical and economic review of secukinumab for moderate-to-severe plaque psoriasis. Expert Rev. Pharm. Outcomes Res. 2016, 16, 153–166. [Google Scholar] [CrossRef]
- Blegvad, C.; Skov, L.; Zachariae, C. Ixekizumab for the treatment of psoriasis: An update on new data since first approval. Expert Rev. Clin. Immunol. 2018, 15, 111–121. [Google Scholar] [CrossRef]
- Rahman, M.; Alam, K.; Ahmad, M.Z.; Gupta, G.; Afzal, M.; Akhter, S.; Kazmi, I.; Jyoti; Ahmad, F.J.; Anwar, F. Classical to current approach for treatment of psoriasis: A review. Endocrine Metab. Immune Disord. Drug Targets 2012, 12, 287–302. [Google Scholar] [CrossRef]
- Xie, J.; Huang, S.; Huang, H.; Deng, X.; Yue, P.; Lin, J.; Yang, M.; Han, L.; Zhang, D.-K. Advances in the Application of Natural Products and the Novel Drug Delivery Systems for Psoriasis. Front. Pharmacol. 2021, 12, 644952. [Google Scholar] [CrossRef]
- Lee, H.; Ha, H.; Lee, J.K.; Seo, C.-S.; Lee, N.-H.; Jung, D.-Y.; Park, S.-J.; Shin, H.K. The Fruits of Cudrania tricuspidata Suppress Development of Atopic Dermatitis in NC/Nga Mice. Phytother. Res. 2011, 26, 594–599. [Google Scholar] [CrossRef]
- Xin, L.-T.; Yue, S.-J.; Fan, Y.-C.; Wu, J.-S.; Yan, D.; Guan, H.-S.; Wang, C.-Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef] [Green Version]
- Uttarkar, S.; Brembilla, N.C.; Boehncke, W.-H. Regulatory cells in the skin: Pathophysiologic role and potential targets for anti-inflammatory therapies. J. Allergy Clin. Immunol. 2019, 143, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Kim, N.; Lee, H.; Woo, E.-R.; Kim, Y.-C.; Oh, H.; Lee, D.-S. Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 7472. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lu, X.; Gan, L.; Zhang, Q.; Lin, L. Xanthones, A Promising Anti-Inflammatory Scaffold: Structure, Activity, and Drug Likeness Analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-Y.; Kim, E.-N.; Jeong, G.-S. Isolation and Quantitative Analysis of Cudratricusxanthone A and Cudraxanthone D from Roots of Cudrania tricuspidata Bureau. Korean J. Pharmacogn. 2019, 50, 59–64. [Google Scholar]
- Van Der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Feldman, S.R.; Krueger, G.G. Psoriasis assessment tools in clinical trials. Ann. Rheum. Dis. 2005, 64, ii65–ii68. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Sah, S.K.; Yang, H.S.; Lee, J.H.; Shin, J.; Kim, T.-Y. Rhododendrin inhibits toll-like receptor-7-mediated psoriasis-like skin inflammation in mice. Exp. Mol. Med. 2017, 49, e349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilek, N.; Dilek, A.R.; Taşkın, Y.; Erkinüresin, T.; Yalçın, Ö.; Saral, Y. Contribution of myeloperoxidase and inducible nitric oxide synthase to pathogenesis of psoriasis. Postepy Dermatol. Alergol. 2016, 33, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Kyriakou, A.; Patsatsi, A.; Vyzantiadis, T.-A.; Sotiriadis, D. Serum Levels of TNF-α, IL-12/23p40, and IL-17 in Plaque Psoriasis and Their Correlation with Disease Severity. J. Immunol. Res. 2014, 2014, 467541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.-C.; Cheng, W.-J.; Korinek, M.; Lin, C.-Y.; Hwang, T.-L. Neutrophils in Psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef]
- Kupczyk, M.; Bocheńska-Marciniak, M.; Górski, P.; Kuna, P. [Myeloperoxidase (MPO) as a marker of neutrophil influx into nasal mucosa after recombinant IL-8 challenge]. Pneumonol. Alergol. Polska 2002, 70, 544–549. [Google Scholar]
- Abdel-Salam, B.K.; Ebaid, H. Expression of CD11b and CD18 on polymorphonuclear neutrophils stimulated with interleukin-2. Cent. Eur. J. Immunol. 2014, 39, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Boivin, G.; Faget, J.; Ancey, P.-B.; Gkasti, A.; Mussard, J.; Engblom, C.; Pfirschke, C.; Contat, C.; Pascual, J.; Vazquez, J.; et al. Durable and controlled depletion of neutrophils in mice. Nat. Commun. 2020, 11, 2762. [Google Scholar] [CrossRef]
- Icen, M.; Crowson, C.S.; McEvoy, M.T.; Dann, F.J.; Gabriel, S.E.; Kremers, H.M. Trends in incidence of adult-onset psoriasis over three decades: A population-based study. J. Am. Acad. Dermatol. 2009, 60, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [Green Version]
- Uva, L.L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of Action of Topical Corticosteroids in Psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [Green Version]
- Shenefelt, P.D. Chapter 18 herbal treatment for dermatologic disorders. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 383. [Google Scholar]
- Lee, H.-J.; Park, S.-E.; Kim, S. Cudrania tricuspidata Fruit Extract Ameliorates Free Fatty Acid-induced Lipid Accumulation in HepG2 Cells. J. Life Sci. 2019, 29, 1144–1151. [Google Scholar]
- Lee, H.-J.; Jue, S.-S.; Kang, S.-K.; Bae, W.-J.; Kim, Y.-C.; Kim, E.-C. Cudraxanthone H Induces Growth Inhibition and Apoptosis in Oral Cancer Cells via NF-κB and PIN1 Pathways. Am. J. Chin. Med. 2015, 43, 1439–1452. [Google Scholar] [CrossRef]
- Jeong, G.-S.; An, R.-B.; Pae, H.-O.; Chung, H.-T.; Yoon, K.-H.; Kang, D.-G.; Lee, H.-S.; Kim, Y.-C. Cudratricusxanthone A Protects Mouse Hippocampal Cells against Glutamate-Induced Neurotoxicity via the Induction of Heme Oxygenase-1. Planta Med. 2008, 74, 1368–1373. [Google Scholar] [CrossRef]
- Park, K.H.; Park, Y.-D.; Han, J.-M.; Im, K.-R.; Lee, B.W.; Jeong, I.Y.; Jeong, T.-S.; Lee, W.S. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorganic Med. Chem. Lett. 2006, 16, 5580–5583. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-B.; Kang, H.-M.; Park, D.-B.; Park, B.-S.; Kim, I.-R. Cudraxanthone D Regulates Epithelial-Mesenchymal Transition by Autophagy Inhibition in Oral Squamous Cell Carcinoma Cell Lines. Evid. -Based Complement. Altern. Med. 2019, 2019, 5213028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeen, M.; Boisgard, A.-S.; Danoy, A.; El Kholti, N.; Salvi, J.-P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model. Pharmaceutics 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Michalak-Stoma, A.; Bartosińska, J.; Kowal, M.; Juszkiewicz-Borowiec, M.; Gerkowicz, A.; Chodorowska, G. Serum Levels of Selected Th17 and Th22 Cytokines in Psoriatic Patients. Dis. Markers 2013, 35, 625–631. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Weigert, C.; Röcken, M. Immunopathogenesis and role of T cells in psoriasis. Clin. Dermatol. 2007, 25, 574–580. [Google Scholar] [CrossRef]
- Nunes, A.; Marto, J.; Gonçalves, L.M.; Simões, S.; Félix, R.; Ascenso, A.; Lopes, F.; Ribeiro, H.M. Novel and Modified Neutrophil Elastase Inhibitor Loaded in Topical Formulations for Psoriasis Management. Pharmaceutics 2020, 12, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Del Marmol, V. Psoriasis: Keratinocytes or Immune Cells—Which Is the Trigger? Dermatology 2018, 235, 91–100. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Park, H.; Pesu, M.; Borie, D.; Changelian, P. New strategies for immunosuppression: Interfering with cytokines by targeting the Jak/Stat pathway. Curr. Opin. Rheumatol. 2005, 17, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Lee, S.; Kang, J.; Choi, Y.-A.; Jang, Y.H.; Jeong, G.-S.; Kim, S.-H. Cudraxanthone D Ameliorates Psoriasis-like Skin Inflammation in an Imiquimod-Induced Mouse Model via Inhibiting the Inflammatory Signaling Pathways. Molecules 2021, 26, 6086. https://doi.org/10.3390/molecules26196086
Kim N, Lee S, Kang J, Choi Y-A, Jang YH, Jeong G-S, Kim S-H. Cudraxanthone D Ameliorates Psoriasis-like Skin Inflammation in an Imiquimod-Induced Mouse Model via Inhibiting the Inflammatory Signaling Pathways. Molecules. 2021; 26(19):6086. https://doi.org/10.3390/molecules26196086
Chicago/Turabian StyleKim, Namkyung, Soyoung Lee, Jinjoo Kang, Young-Ae Choi, Yong Hyun Jang, Gil-Saeng Jeong, and Sang-Hyun Kim. 2021. "Cudraxanthone D Ameliorates Psoriasis-like Skin Inflammation in an Imiquimod-Induced Mouse Model via Inhibiting the Inflammatory Signaling Pathways" Molecules 26, no. 19: 6086. https://doi.org/10.3390/molecules26196086