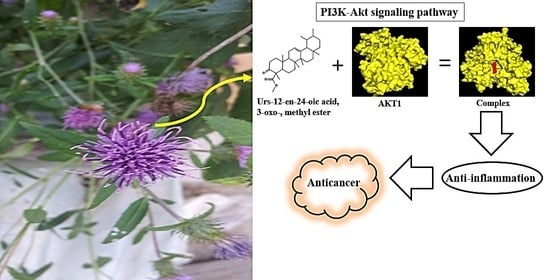
Network Pharmacology-Based Study to Uncover Potential Pharmacological Mechanisms of Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.) Flower against Cancer
Abstract
:1. Introduction
2. Results
2.1. Bioactives from C. maackii Flower
2.2. Overlapping Targets between SEA and STP Associated with Bioactives
2.3. Overlapping Targets between Cancer-Associated Targets and the Final 51 Overlapping Targets
2.4. Acquisition of a Hub Target from PPI Networks
2.5. Identification of a Hub Signaling from Bubble Chart
2.6. S–T–B Network Analysis of C. maackii Flower against Cancer
2.7. MDT of 3 Targets and 10 Bioactives Connected to PI3K-Akt Signaling Pathway
2.8. Comparative Analysis of MDT against Positive Controls on a Hub Target
2.9. Toxicological Properties of a Selected Key Bioactive
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Identification
4.2. Plant Preparation, Extraction
4.3. GC-MS Analysis Condition
4.4. Bioactives Database Construction and Drug-Likeness Property
4.5. Target Targets Related to Selected Bioactives or Cancer
4.6. Construction of PPI Networks and Bubble Chart
4.7. Construction of a Size Map on S-T-B Network
4.8. Preparation for MDT of Targets
4.9. Preparation for MDT of Positive Standard Ligands
4.10. Preparation for MDT of Ligand Molecules
4.11. Ligand-Protein Docking
4.12. Toxicological Properties Prediction by admetSAR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ADME | Absorption: Distribution, Metabolism, Excretion; |
| AGE | Advanced Glycation End-product; |
| AMPK | AMP-activated protein kinase; |
| cAMP | Cyclic AMP; |
| C. maackii | Cirsium japonicum var. maackii (Maxim.) Matsum.: C. maackii; |
| DLCs | Drug-Like Compounds; |
| BCR | B cell receptor; |
| GC-MS | Gas Chromatography Mass Spectrum; |
| HIF-1 | Hypoxia Inducible Factor-1; |
| KEGG | Kyoto Encyclopedia of Genes and Genomes; |
| MAPK | Mitogen Activated Protein Kinase; |
| MDT | Molecular Docking Test; |
| NF- κB | Nuclear Factor Kappa B; |
| OMIM | Online Mendelian Inheritance in Man; |
| PI3K-Akt | Phosphoinositide 3-kinase-Akt; |
| PLD | Phospholipase D; |
| PPAR | Peroxisome Proliferator Activated Receptor; |
| PPI | Protein-protein interaction; |
| RAGE | Advanced Glycation End-product Receptor; |
| Rap1 | Ras-associated protein-1; |
| ROS | Reactive Oxygen Species; |
| SEA | Similarity Ensemble Approach; |
| SMILES | Simplified Molecular Input Line Entry System; |
| S-T-B | Signaling pathway-Target protein-Bioactive; |
| STP | SwissTargetPrediction; |
| t-BHP | Tert-butyl hydroperoxide; |
| TTD | Therapeutic Target Database; |
| VEGF | Vascular Endothelial Growth Factor |
References
- Bertram, J.S. The molecular biology of cancer. Mol. Asp. Med. 2000, 21, 167–223. [Google Scholar] [CrossRef]
- Nakad, R.; Schumacher, B. DNA Damage Response and Immune Defense: Links and Mechanisms. Front. Genet. 2016, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Welsh, C.; Day, R.; McGurk, C.; Masters, J.R.; Wood, R.D.; Köberle, B. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. Int. J. Cancer 2004, 110, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacourt, T.E.; Vichaya, E.G.; Chiu, G.S.; Dantzer, R.; Heijnen, C.J. The high costs of low-grade inflammation: Persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front. Behav. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Rayburn, E.R.; Ezell, S.J.; Zhang, R. Anti-inflammatory agents for cancer therapy. Mol. Cell. Pharmacol. 2009, 1, 29–43. [Google Scholar] [CrossRef]
- Subramaniam, M.; Liew, S.K.; In, L.L.; Awang, K.; Ahmed, N.; Nagoor, N.H. Inactivation of nuclear factor κB by MIP-based drug combinations augments cell death of breast cancer cells. Drug Des. Dev. Ther. 2018, 12, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Shewach, D.S.; Kuchta, R.D. Introduction to Cancer Chemotherapeutics. Chem. Rev. 2009, 109, 2859–2861. [Google Scholar] [CrossRef] [Green Version]
- Kayl, A.E.; Meyers, C.A. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr. Opin. Obstet. Gynecol. 2006, 18, 24–28. [Google Scholar] [CrossRef]
- Aravindaram, K.; Yang, N.-S. Anti-Inflammatory Plant Natural Products for Cancer Therapy. Planta Medica 2010, 76, 1103–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, M.; Rahman, P. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagle, A.; Seong, S.H.; Shrestha, S.; Jung, H.A.; Choi, J.S. Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease. Molecules 2019, 24, 649. [Google Scholar] [CrossRef] [Green Version]
- Nazaruk, J.; Wajs-Bonikowska, A.; Bonikowski, R. Components and antioxidant activity of fruits of Cirsium palustre and C. rivulare. Chem. Nat. Compd. 2012, 48, 8–10. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Jho, H.-K.; Kim, N.-I.; Rhyu, M.R. Cirsium japonicum elicits endothelium-dependent relaxation via histamine H1-receptor in rat thoracic aorta. J. Ethnopharmacol. 2008, 116, 223–227. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Li, D.; Liu, W.; Luo, X.; Zhang, R.; Li, L.; Zhao, J. Anticancer activity and quantitative analysis of flavone of Cirsium japonicum DC. Nat. Prod. Res. 2007, 21, 915–922. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, Y.; Luo, B.; Liu, W.; Wei, R.; Yang, C.; Ding, C.; Xu, X.; He, M. Hepatoprotective phenylethanoid glycosides from Cirsium setosum. Nat. Prod. Res. 2015, 30, 1824–1829. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.-H.; Lee, H.-J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDA-MB-231 breast cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Abdul, Q.A.; Byun, J.S.; Joung, E.-J.; Gwon, W.-G.; Lee, M.-S.; Kim, H.-R.; Choi, J.S. Protective effects of flavonoids isolated from Korean milk thistle Cirsium japonicum var. maackii (Maxim.) Matsum on tert-butyl hydroperoxide-induced hepatotoxicity in HepG2 cells. J. Ethnopharmacol. 2017, 209, 62–72. [Google Scholar] [CrossRef]
- Gartung, A.; Yang, J.; Sukhatme, V.P.; Bielenberg, D.R.; Fernandes, D.; Chang, J.; Schmidt, B.A.; Hwang, S.H.; Zurakowski, D.; Huang, S.; et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc. Natl. Acad. Sci. USA 2019, 116, 1698–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatarrai, G.; Seong, S.H.; Jung, H.A.; Choi, J.S. Isolation and Quantitative Analysis of BACE1 Inhibitory Compounds from Cirsium maackii Flower. Nat. Prod. Sci. 2019, 25, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Aral, H.; Aral, T.; Çelik, K.S.; Ziyadanoğulları, B.; Ziyadanoğulları, R. HPLC Separation of Different Groups of Small Polar Compounds on a Novel Amide-Embedded Stationary Phase. Chromatographia 2014, 77, 771–781. [Google Scholar] [CrossRef]
- De La Torre, M.P.D.; Priego-Capote, F.; De Castro, M.D.L. Tentative identification of polar and mid-polar compounds in extracts from wine lees by liquid chromatography-tandem mass spectrometry in high-resolution mode. J. Mass Spectrom. 2015, 50, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sympli, H.D. Estimation of drug-likeness properties of GC–MS separated bioactive compounds in rare medicinal Pleione maculata using molecular docking technique and SwissADME in silico tools. Netw. Model. Anal. Heal. Informatics Bioinform. 2021, 10, 1–36. [Google Scholar] [CrossRef]
- Oh, K.K.; Adnan, M.; Cho, D.H. Network pharmacology of bioactives from Sorghum bicolor with targets related to diabetes mellitus. PLoS ONE 2020, 15, e0240873. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Feng, S.; Wang, J.; Zhang, F. Target recognition and network pharmacology for revealing anti-diabetes mechanisms of natural product. J. Comput. Sci. 2020, 45, 101186. [Google Scholar] [CrossRef]
- Ye, H.; Wei, J.; Tang, K.; Feuers, R.; Hong, H. Drug Repositioning Through Network Pharmacology. Curr. Top. Med. Chem. 2016, 16, 3646–3656. [Google Scholar] [CrossRef]
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Mäkelä, S.; Aittokallio, T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef]
- Nisa, S.; Khan, N.; Shah, W.; Sabir, M.; Khan, W.; Bibi, Y.; Jahangir, M.; Haq, I.U.; Alam, S.; Qayyum, A. Identification and Bioactivities of Two Endophytic Fungi Fusarium fujikuroi and Aspergillus tubingensis from Foliar Parts of Debregeasia salicifolia. Arab. J. Sci. Eng. 2020, 45, 4477–4487. [Google Scholar] [CrossRef]
- Bassole, I.H.N.; Nebie, R.; Savadogo, A.; Ouattara, C.T.; Barro, N.; Traore, S.A. Composition and antimicrobial activities of the leaf and flower essential oils of Lippia chevalieri and Ocimum canum from Burkina Faso. Afr. J. Biotechnol. 2005, 4, 1156–1160. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, H.-J.; Jeong, J.-A.; Jung, J.-W. Palmitic acid inhibits inflammatory responses in lipopolysaccharide-stimulated mouse peritoneal macrophages. Orient. Pharm. Exp. Med. 2010, 10, 37–43. [Google Scholar] [CrossRef]
- Sebastianes, F.L.S.; Cabedo, N.; El Aouad, N.; Valente, A.M.M.P.; Lacava, P.T.; Azevedo, J.L.; Pizzirani-Kleiner, A.A.; Cortes, D. 3-Hydroxypropionic Acid as an Antibacterial Agent from Endophytic Fungi Diaporthe phaseolorum. Curr. Microbiol. 2012, 65, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.M.; Solano, A.G.R.; Prado, M.A.F.; Nunan, E.D.A. Investigation of fatty acid esters to replace isopropyl myristate in the sterility test for ophthalmic ointments. J. Pharm. Biomed. Anal. 2006, 42, 630–634. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.; Webb, H.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.; Mainwaring, D.E.; et al. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef]
- Maruthi, R.; Chandan, R.; Barath, M.; Datta, G.N.; D’Silva, M.; Kumari, M.K.; Ahmad, F.; Geetha, R. Analytical method development and validation of teneligliptin by rp-uflc. Res. J. Pharm. Technol. 2020, 13, 4035. [Google Scholar] [CrossRef]
- PCIDB. Available online: https://www.genome.jp/db/pcidb (accessed on 19 March 2021).
- Pédeboscq, S.; Rey, C.; Petit, M.; Harpey, C.; De Giorgi, F.; Ichas, F.; Lartigue, L. Non-Antioxidant Properties of α-Tocopherol Reduce the Anticancer Activity of Several Protein Kinase Inhibitors In Vitro. PLoS ONE 2012, 7, e36811. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.K.; Adnan, M.; Ju, I.; Cho, D.H. A network pharmacology study on main chemical compounds from Hibiscus cannabinus L. leaves. RSC Adv. 2021, 11, 11062–11082. [Google Scholar] [CrossRef]
- Zhong, R.-F.; Xu, G.-B.; Wang, Z.; Wang, A.-M.; Guan, H.-Y.; Li, J.; He, X.; Liu, J.-H.; Zhou, M.; Li, Y.-J.; et al. Identification of anti-inflammatory constituents from Kalimeris indica with UHPLC-ESI-Q-TOF-MS/MS and GC–MS. J. Ethnopharmacol. 2015, 165, 39–45. [Google Scholar] [CrossRef]
- Wonkam, A.K.N.; Ngansop, C.A.N.; Wouamba, S.C.N.; Jouda, J.B.; Happi, G.M.; Boyom, F.F.; Sewald, N.; Lenta, B.N. Rothmanniamide and other constituents from the leaves of Rothmannia hispida (K.Schum.) fagerl. (Rubiaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2020, 93, 104137. [Google Scholar] [CrossRef]
- Kühnl, A.; Musiol, A.; Heitzig, N.; Johnson, D.E.; Ehrhardt, C.; Grewal, T.; Gerke, V.; Ludwig, S.; Rescher, U. Late Endosomal/Lysosomal Cholesterol Accumulation Is a Host Cell-Protective Mechanism Inhibiting Endosomal Escape of Influenza A Virus. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.; Wilson, M.; Gorham, R.; Harrison, R.E.S.; Morikis, V.A.; Kieslich, C.; Orr, A.A.; Coley, A.V.; Tamamis, P.; Morikis, D. Virtual Screening of Chemical Compounds for Discovery of Complement C3 Ligands. ACS Omega 2018, 3, 6427–6438. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-X.; Liu, Z.-H.; Jiang, H.-C.; Qi, S.-Y.; Zhang, W.-H.; Zhu, A.-L.; Wang, X.-Q.; Wu, M. Overexpression of Akt-1 gene in human hepatocellular carcinoma. Chin. J. Cancer Res. 2002, 14, 161–164. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Fernandez-Hernando, C.; Cirino, G.; Sessa, W.C. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc. Natl. Acad. Sci. USA 2009, 106, 14552–14557. [Google Scholar] [CrossRef] [Green Version]
- Chenniappan, J.; Sankaranarayanan, A.; Arjunan, S. Evaluation of Antimicrobial Activity of Cissus quadrangularis L. stem extracts against Avian Pathogens and Determination of its Bioactive Constituents using GC-MS. J. Sci. Res. 2020, 64, 90–96. [Google Scholar] [CrossRef]
- Sharma, T.; Jana, S. Boswellic acids as natural anticancer medicine: Precious gift to humankind. J. Herb. Med. 2020, 20, 100313. [Google Scholar] [CrossRef]
- Fuentes, E.; Guzmán-Jofre, L.; Moore-Carrasco, R.; Palomo, I. Role of PPARs in inflammatory processes associated with metabolic syndrome (Review). Mol. Med. Rep. 2013, 8, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Grabacka, M.; Reiss, K. Anticancer Properties of PPARα-Effects on Cellular Metabolism and Inflammation. PPAR Res. 2008, 2008, 930705. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. In Proceedings of the Biochimica et Biophysica Acta—Proteins and Proteomics, Warsaw, Poland, 25–29 June 2005; pp. 253–262. [Google Scholar]
- Qian, C.; Qi, Y.; Zhong, S.; Zeng, J.; Chen, X.; Yao, J. Mitogen-activated protein kinase inhibition enhances the antitumor effects of sporamin in human pancreatic cancer cells. Oncol. Lett. 2018, 16, 1237–1242. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Sukhova, G.K.; Wong, H.K.; Xu, A.; Tergaonkar, V.; Vanhoutte, P.M.; Tang, E.H.C. Rap1 induces cytokine production in pro-inflammatory macrophages through NFκB signaling and is highly expressed in human atherosclerotic lesions. Cell Cycle 2015, 14, 3580–3592. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Ye, G.; Liu, L.; Wei, L. The downregulation of Rap1 GTPase-activating protein is associated with a poor prognosis in colorectal cancer and may impact on tumor progression. Oncol. Lett. 2018, 15, 7661–7668. [Google Scholar] [CrossRef]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Bong, A.H.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 1786–1794. [Google Scholar] [CrossRef]
- Erdogan, S.; Aslantas, O.; Celik, S.; Atik, E. The effects of increased cAMP content on inflammation, oxidative stress and PDE4 transcripts during Brucella melitensis infection. Res. Vet. Sci. 2008, 84, 18–25. [Google Scholar] [CrossRef]
- Zou, T.; Liu, J.; She, L.; Chen, J.; Zhu, T.; Yin, J.; Li, X.; Li, X.; Zhou, H.; Liu, Z. A perspective profile of ADCY1 in cAMP signaling with drug-resistance in lung cancer. J. Cancer 2019, 10, 6848–6857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, J.M.; Velloso, L.A. Hypoxia inducible factor as a central regulator of metabolism—Implications for the development of obesity. Front. Neurosci. 2018, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today 2007, 12, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.F. Sphingolipids in inflammation: Pathological implications and potential therapeutic targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnusamy, S.; Meyers-Needham, M.; Senkal, C.E.; Saddoughi, S.A.; Sentelle, D.; Selvam, S.P.; Salas, A.; Ogretmen, B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Futur. Oncol. 2010, 6, 1603–1624. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.W.; Choi, K.-Y.; Min, D.S. Functional Regulation of Phospholipase D Expression in Cancer and Inflammation. J. Biol. Chem. 2014, 289, 22575–22582. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Han, J.-S. Phospholipase D and Its Essential Role in Cancer. Mol. Cells 2017, 40, 805–813. [Google Scholar] [CrossRef]
- Hawkins, P.; Stephens, L. PI3K signalling in inflammation. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 882–897. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 2018, 83, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-C.; Chou, C.-C.; Kulp, S.; Chen, C.-S. AMPK as a Potential Anticancer Target—Friend or Foe? Curr. Pharm. Des. 2014, 20, 2607–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-H.; Wei, Y.; Lao, X.-M.; Chen, D.-P.; Huang, C.-X.; Lin, Q.-Y.; He, M.; Liao, Y.; Zheng, L.; Li, B.; et al. B cells polarize pathogenic inflammatory T helper subsets through ICOSL-dependent glycolysis. Sci. Adv. 2020, 6, eabb6296. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef]
- Luiten, R.M.; Warnaar, S.O.; Schuurman, J.; Pasmans, S.G.; Latour, S.; Daëron, M.; Fleuren, G.J.; Litvinov, S.V. Chimeric immunoglobulin E reactive with tumor-associated antigen activates human FcεRI bearing cells. Hum. Antibodies 1997, 8, 169–180. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin Resistance and Cancer Risk: An Overview of the Pathogenetic Mechanisms. Exp. Diabetes Res. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen Signaling in Metabolic Inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef] [Green Version]
- Orbach, H.; Shoenfeld, Y. Hyperprolactinemia and autoimmune diseases. Autoimmun. Rev. 2007, 6, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sethi, B.K.; Chanukya, G.; Nagesh, V.S. Prolactin and cancer: Has the orphan finally found a home? Indian J. Endocrinol. Metab. 2012, 16, S195–S198. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, A.H.; Fliers, E.; Boelen, A. Thyroid hormone in inflammation. Endocr. Abstr. 2019, 65. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Yu, X. Bone Marrow Adipocytes, Adipocytokines, and Breast Cancer Cells: Novel Implications in Bone Metastasis of Breast Cancer. Front. Oncol. 2020, 10, 561595. [Google Scholar] [CrossRef] [PubMed]
- Thanasupawat, T.; Glogowska, A.; Nivedita-Krishnan, S.; Wilson, B.; Klonisch, T.; Hombach-Klonisch, S. Emerging roles for the relaxin/RXFP1 system in cancer therapy. Mol. Cell. Endocrinol. 2019, 487, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.M.; Storey, K.B. Pro-inflammatory AGE-RAGE signaling is activated during arousal from hibernation in ground squirrel adipose. PeerJ 2018, 6, e4911. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.; Munesue, S.; Harashima, A.; Sato, A.; Shindo, M.; Nakajima, S.; Inada, M.; Tanaka, M.; Takeuchi, A.; Tsuchiya, H.; et al. In vitro anticancer effects of a RAGE inhibitor discovered using a structure-based drug design system. Oncol. Lett. 2018, 15, 4627–4634. [Google Scholar] [CrossRef]
- Oh, K.K.; Adnan, M.; Cho, D.H. Active ingredients and mechanisms of Phellinus linteus (grown on Rosa multiflora) for alleviation of Type 2 diabetes mellitus through network pharmacology. Gene 2021, 768, 145320. [Google Scholar] [CrossRef]
- Keiser, M.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Khanal, P.; Patil, B.M.; Chand, J.; Naaz, Y. Anthraquinone Derivatives as an Immune Booster and their Therapeutic Option Against COVID-19. Nat. Prod. Bioprospect. 2020, 10, 325–335. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. ACS Pub. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2018, 35, 1067–1069. [Google Scholar] [CrossRef]








| No. | Compounds | Pubchem ID | RT (mins) | Area (%) | Pharmacological Activities (References) |
|---|---|---|---|---|---|
| 1 | Glyceraldehyde | 751 | 3.510 | 0.91 | No reported |
| 2 | 6-Methyluracil | 12283 | 4.279 | 0.88 | No reported |
| 3 | 3-Hydroxy-2,3-dihydromaltol | 119838 | 4.789 | 5.19 | No reported |
| 4 | Pyranopyridine | 534033 | 5.414 | 0.69 | No reported |
| 5 | Formicin | 69365 | 6.096, 6.212 | 0.58 | No reported |
| 6 | 4-Nitro-2-picoline N-oxide | 95291 | 6.721 | 0.6 | No reported |
| 7 | 2-Vinyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | 135493011 | 6.933 | 1.35 | Antibacterial and antifungal activity [32] |
| 8 | 2-Chloromethyl-3,3-dichloropropene | 43492 | 7.058 | 0.51 | No reported |
| 9 | d-Lyxo-d-manno-nononic-1,4-lactone | 535556 | 7.183 | 0.43 | No reported |
| 10 | Pentyl isobutyrate | 75554 | 7.327 | 0.26 | Antimicrobial activity [33] |
| 11 | 2-Methyl-3-nitrosooxazolidine | 38357 | 7.673 | 0.23 | No reported |
| 12 | N-Nitrosomethylethanolamine | 33646 | 7.808 | 2.71 | No reported |
| 13 | 1,4,7,10-Tetraoxacyclododecan-2-one | 533646 | 8.145 | 0.15 | No reported |
| 14 | 4-methylpyrimidin-2-ol | 407091 | 8.231 | 0.58 | No reported |
| 15 | Diethyl malonate | 7761 | 8.750 | 0.46 | No reported |
| 16 | Palmitic acid | 985 | 8.943, 9.443, 9.760, 10.106 | 4.03 | Anti-inflammation [34] |
| 17 | Methyl 3,6-anhydrohexopyranoside # | 91691384 | 9.154 | 0.65 | No reported |
| 18 | 3-Hydroxypropionic acid | 68152 | 9.625 | 1.17 | Antibacterial activity [35] |
| 19 | 2,2,4-Trichloro-1,3-cyclopentenedione | 150757 | 9.846 | 0.65 | No reported |
| 20 | Isopropyl palmitate | 8907 | 10.673 | 0.54 | Antibacterial activity [36] |
| 21 | Stearic acid | 5281 | 10.933 | 0.72 | Antibacterial activity [37] |
| 22 | Pregn-5,7-diene-3-ol-20-one | 21117403 | 11.068 | 0.25 | No reported |
| 23 | 9-Heptadecanone | 10887 | 11.173 | 0.95 | Antibacterial activity [38] |
| 24 | Allyl stearate | 80500 | 12.087 | 1.05 | No reported |
| 25 | Squalene | 638072 | 12.250 | 0.54 | Anti-oxidant [39] |
| 26 | Prexanthoperol | 628742 | 13.952 | 13.65 | No reported |
| 27 | α-Tocopherol | 14985 | 14.664 | 0.94 | Anticancer [40] |
| 28 | β-Stigmasterol | 6432745 | 16.289 | 1.68 | Anti-inflammation [39] |
| 29 | 4,4-Dimethylcholest-7-en-3-ol | 5460076 | 17.077 | 1.06 | No reported |
| 30 | Aristol-9-en-8-one # | 6432651 | 17.837 | 1.11 | No reported [39] |
| 31 | α-Amyrin | 73170 | 18.587 | 2.53 | Anti-obesity [41] |
| 32 | Urs-12-en-24-oic acid, 3-oxo-, methyl ester | 612822 | 19.125 | 9.04 | Potential Anti-inflammation [42] |
| 33 | Lupenyl acetate | 323074 | 20.019 | 24.42 | Antibacterial activity [43] |
| 34 | Cholesterol | 5997 | 21.693, 21.952 | 15.19 | Protective cell against pathogens [44] |
| Compounds | Lipinski Rules | Lipinski’s Violations | Bioavailability Score | TPSA (Å2) | ||||
|---|---|---|---|---|---|---|---|---|
| MW | HBA | HBD | MLog P | |||||
| No. | <500 | <10 | ≤5 | ≤4.15 | ≤1 | >0.1 | <140 | |
| 1 | Glyceraldehyde | 90.08 | 3 | 2 | −1.66 | 0 | 0.55 | 57.53 |
| 2 | 6-Methyluracil | 126.11 | 2 | 2 | −0.39 | 0 | 0.55 | 65.72 |
| 3 | 3-Hydroxy-2,3-dihydromaltol | 144.13 | 4 | 2 | −1.77 | 0 | 0.85 | 66.76 |
| 4 | Pyranopyridine | 133.15 | 2 | 0 | 0.73 | 0 | 0.55 | 22.12 |
| 5 | Formicin | 89.09 | 2 | 2 | −0.85 | 0 | 0.55 | 49.33 |
| 6 | 4-Nitro-2-picoline N-oxide | 154.12 | 3 | 0 | −0.15 | 0 | 0.55 | 71.28 |
| 7 | 2-Vinyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | 294.26 | 7 | 4 | −1.77 | 0 | 0.55 | 133.49 |
| 8 | 2-Chloromethyl-3,3-dichloropropene | 159.44 | 0 | 0 | 2.81 | 0 | 0.55 | 0.00 |
| 9 | d-Lyxo-d-manno-nononic-1,4-lactone | 268.22 | 9 | 7 | −3.85 | 1 | 0.55 | 167.91 |
| 10 | Pentyl isobutyrate | 158.24 | 2 | 0 | 2.28 | 0 | 0.55 | 26.30 |
| 11 | 2-Methyl-3-nitrosooxazolidine | 116.12 | 3 | 0 | −0.42 | 0 | 0.55 | 41.90 |
| 12 | N-Nitrosomethylethanolamine | 104.11 | 3 | 1 | −0.89 | 0 | 0.55 | 52.90 |
| 13 | 1,4,7,10-Tetraoxacyclododecan-2-one | 190.19 | 5 | 0 | −0.99 | 0 | 0.55 | 53.99 |
| 14 | 4-methylpyrimidin-2-ol | 110.11 | 2 | 1 | −0.27 | 0 | 0.55 | 45.75 |
| 15 | Diethyl malonate | 160.17 | 4 | 0 | 0.6 | 0 | 0.55 | 52.60 |
| 16 | Palmitic acid | 256.42 | 2 | 1 | 4.19 | 1 | 0.85 | 37.30 |
| 17 | Methyl 3,6-anhydrohexopyranoside # | 176.17 | 5 | 2 | −1.59 | 0 | 0.55 | 68.15 |
| 18 | 3-Hydroxypropionic acid | 90.08 | 3 | 2 | −0.85 | 0 | 0.85 | 57.53 |
| 19 | 2,2,4-Trichloro-1,3-cyclopentenedione | 199.42 | 2 | 0 | 0.55 | 0 | 0.55 | 34.14 |
| 20 | Isopropyl palmitate | 298.5 | 2 | 0 | 4.91 | 1 | 0.55 | 26.30 |
| 21 | Stearic acid | 284.48 | 2 | 1 | 4.67 | 1 | 0.85 | 37.30 |
| 22 | Pregn-5,7-diene-3-ol-20-one | 314.46 | 2 | 1 | 3.95 | 0 | 0.55 | 37.30 |
| 23 | 9-Heptadecanone | 254.45 | 1 | 0 | 4.55 | 1 | 0.55 | 17.07 |
| 24 | Allyl stearate | 324.54 | 2 | 0 | 5.25 | 1 | 0.55 | 26.30 |
| 25 | Squalene | 410.72 | 0 | 0 | 7.93 | 1 | 0.55 | 0.00 |
| 26 | Prexanthoperol | 314.42 | 3 | 1 | 2.94 | 0 | 0.55 | 54.37 |
| 27 | Vitamin E | 430.71 | 2 | 1 | 6.14 | 1 | 0.55 | 29.46 |
| 28 | β-Stigmasterol | 412.69 | 1 | 1 | 6.62 | 1 | 0.55 | 20.23 |
| 29 | 4,4-Dimethylcholest-7-en-3-ol | 414.71 | 1 | 1 | 6.73 | 1 | 0.55 | 20.23 |
| 30 | Aristol-9-en-8-one # | 218.33 | 1 | 0 | 3.56 | 0 | 0.55 | 17.07 |
| 31 | α-Amyrin | 426.72 | 1 | 1 | 6.92 | 1 | 0.55 | 20.23 |
| 32 | Urs-12-en-24-oic acid, 3-oxo-, methyl ester | 468.71 | 3 | 0 | 5.92 | 1 | 0.55 | 43.37 |
| 33 | Lupenyl acetate | 144.88 | 2 | 0 | 7.08 | 1 | 0.55 | 26.30 |
| 34 | Cholesterol | 386.65 | 1 | 1 | 6.34 | 1 | 0.55 | 20.23 |
| No. | Target | Degree | No. | Target | Degree |
|---|---|---|---|---|---|
| 1 | AKT1 | 29 | 24 | MGLL | 5 |
| 2 | VEGFA | 21 | 25 | S1PR1 | 5 |
| 3 | ESR1 | 16 | 26 | SHH | 5 |
| 4 | AR | 13 | 27 | VDR | 5 |
| 5 | CYP19A1 | 11 | 28 | TOP2A | 5 |
| 6 | PPARG | 11 | 29 | ADORA2B | 4 |
| 7 | IL1B | 10 | 30 | PTGER2 | 4 |
| 8 | CNR1 | 9 | 31 | S1PR3 | 4 |
| 9 | TRPV1 | 9 | 32 | ADORA2A | 3 |
| 10 | PPARA | 9 | 33 | CYP24A1 | 3 |
| 11 | PTPN1 | 9 | 34 | FOLH1 | 3 |
| 12 | HMGCR | 8 | 35 | PTPN6 | 3 |
| 13 | ESR2 | 7 | 36 | CA2 | 2 |
| 14 | PRKCA | 7 | 37 | FABP3 | 2 |
| 15 | ABCB1 | 6 | 38 | NR1H3 | 2 |
| 16 | CDC25B | 6 | 39 | PHLPP1 | 2 |
| 17 | PTGER4 | 6 | 40 | PTPRF | 2 |
| 18 | SHBG | 6 | 41 | PRKCE | 2 |
| 19 | CYP17A1 | 6 | 42 | PTGFR | 2 |
| 20 | SRD5A2 | 6 | 43 | CA1 | 1 |
| 21 | ADORA3 | 5 | 44 | FNTB | 1 |
| 22 | AKR1B1 | 5 | 45 | NR1H4 | 1 |
| 23 | CDC25A | 5 | 46 | IMPDH2 | 1 |
| No. | Target | Degree | Bioactive | Degree | No. | Target | Degree | Bioactive | Degree |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AKT1 | 18 | Urs-12-en-24-oic acid,3-oxo-, methyl ester | 36 | 13 | S1PR1 | 1 | Cholesterol | 14 |
| 2 | PRKCA | 12 | Palmitic acid | 27 | 14 | S1PR3 | 1 | Pregn-5,7-diene-3-ol-20-one | 10 |
| 3 | VEGFA | 7 | Stearic acid | 26 | 15 | PTPN6 | 1 | Methyl 3,6-anhydro hexopyranoside # | 7 |
| 4 | PPARA | 3 | α-Tocopherol | 26 | 16 | CNR1 | 1 | Aristol-9-en-8-one # | 7 |
| 5 | ESR1 | 3 | Isopropyl palmitate | 26 | 17 | HMGCR | 1 | Squalene | 3 |
| 6 | PPARG | 2 | Allyl stearate | 25 | 18 | PTPN1 | 1 | Lupenyl acetate | 3 |
| 7 | ESR2 | 2 | α-Amyrin | 21 | 19 | PTPRF | 1 | 2-Vinyl-9- [β-d-ribofuranosyl] hypoxanthine | 1 |
| 8 | PRKCE | 2 | 9-Heptadecanone | 21 | 20 | CDC25B | 1 | ||
| 9 | IL1B | 2 | β-Stigmasterol | 20 | 21 | PTGFR | 1 | ||
| 10 | NR1H3 | 1 | 4,4-Dimethyl-cholest-7-en-3-ol | 20 | 22 | ADORA2B | 1 | ||
| 11 | FABP3 | 1 | Pentyl isobutyrate | 17 | 23 | PTGER2 | 1 | ||
| 12 | CYP17A1 | 1 | d-Lyxo-d-manno-nononic-1,4-lactone | 15 | 24 | PHLPP1 | 1 |
| KEGG ID & Description | Targets | False Discovery Rate |
|---|---|---|
| hsa03320: PPAR signaling pathway | PPARA, PPARD, PPARG, NR1H3, FABP3 | 0.0000695 |
| hsa04917: Prolactin signaling pathway | AKT1, ESR1, ESR2, CYP17A1 | 0.00063 |
| hsa04933: AGE-RAGE signaling pathway in diabetic complications | AKT1, VEGFA, PRKCA, PRKCE, IL1B | 0.00018 |
| hsa04370: VEGF signaling pathway | AKT1, VEGFA, PRKCA | 0.0068 |
| hsa04071: Sphingolipid signaling pathway | AKT1, VEGFA, PRKCE, S1PR1, S1PR3 | 0.00027 |
| hsa04066: HIF-1 signaling pathway | AKT1, VEGFA, PRKCA | 0.016 |
| hsa04664: Fc epsilon RI signaling pathway | AKT1, PRKCA | 0.0492 |
| hsa04920: Adipocytokine signaling pathway | AKT1, PPARA | 0.0492 |
| hsa04662: B cell receptor signaling pathway | AKT1, PTPN6 | 0.0492 |
| hsa04919: Thyroid signaling pathway | AKT1, ESR1, PRKCA | 0.0236 |
| hsa04015: Rap1 signaling pathway | AKT1, VEGFA, PRKCA, VEGFA, ADORA2B | 0.003 |
| hsa04152: AMPK signaling pathway | AKT1, PPARG, HMGCR | 0.025 |
| hsa04926: Relaxin signaling pathway | AKT1, VEGFA, PRKCA | 0.0278 |
| hsa04915: Estrogen signaling pathway | AKT1, ESR1, ESR2 | 0.0279 |
| hsa04910: Insulin signaling pathway | AKT1, PTPN1, PTPRF | 0.0279 |
| hsa04072: Phospholipase D signaling pathway | AKT1, PRKCA | 0.0324 |
| hsa04010: MAPK signaling pathway | AKT1, VEGFA, PRKCA, IL1B | 0.0103 |
| hsa04020: Calcium signaling pathway | PRKCA, ADORA2B | 0.0457 |
| hsa04024: cAMP signaling pathway | AKT1, PPARA, PTGER2 | 0.0492 |
| hsa04151: PI3K-Akt signaling pathway | AKT1, VEGFA, PRKCA, PHLPP1 | 0.0487 |
| Hydrogen Bond Interactions | Hydrophobic Interactions | ||||||
|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Binding Energy (kcal/mol) | Amino Acid Residue | R Group(s) Involved in Hydrogen Bonding | Distance (Å) | Amino Acid Residue |
| 5KCV | Urs-12-en-24-oic acid, 3-oxo-, methyl ester | 612822 | −12.8 | N/A | N/A | N/A | Gln59, Lys268, Asn53 |
| Trp80, Leu202, Leu78 | |||||||
| α-Tocopherol | 14985 | −5.8 | Leu78 | R-OH | 2.71 | Ala58, Val270, Lys268 | |
| Val201, Leu202, Gln203 | |||||||
| Trp80, Asn53 | |||||||
| Hydrogen Bond Interactions | Hydrophobic Interactions | ||||||
|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Binding Energy (kcal/mol) | Amino Acid Residue | R Group(s) Involved in Hydrogen Bonding | Distance (Å) | Amino Acid Residue |
| 3V2A | Allyl stearate | 80500 | −5.1 | Phe47 | RCOOR′ | 3.18 | Ile46, Lys48, Lys286 |
| Asp276, Arg275, Pro40 | |||||||
| Phe36 | |||||||
| Isopropyl palmitate | 8907 | −5.1 | N/A | N/A | N/A | Asp276, Asp34, Ile46 | |
| Phe47, Lys48, Lys286 | |||||||
| Phe36, Pro40 | |||||||
| Methyl 3,6-anhydro hexopyranoside # | 91691384 | −5.2 | Ser310, Pro85 | R-O-R′, R-OH | 2.98, 3.23, 2.82 | Lys84, Gln87, Gly312 | |
| Asp257, Gly255, Ile256 | |||||||
| Glu44 | |||||||
| Hydrogen Bond Interactions | Hydrophobic Interactions | ||||||
|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Binding Energy (kcal/mol) | Amino Acid Residue | R Group(s) Involved in Hydrogen Bonding | Distance (Å) | Amino Acid Residue |
| 3IW4 | Allyl stearate | 80500 | −6.2 | Lys 396 | RCOOR′ | 3.21 | Asp395, Leu393, Pro397 |
| Arg608, Pro398, Val664 | |||||||
| Pro666, Ser473, Glu474 | |||||||
| His665, Lys478, Ile667 | |||||||
| Gln402, Asn660 | |||||||
| Isopropyl palmitate | 8907 | −6.3 | Asn660 | RCOOR′ | 2.92 | Asp395, Leu393, Pro397 | |
| Lys396, Gln402,Pro398 | |||||||
| Arg608, Ile667, Pro666 | |||||||
| Val664, His665, Lys478 | |||||||
| N/A | N/A | N/A | Pro502, Gln650, Ile645 | ||||
| Pentyl isobutyrate | 75554 | −5.0 | Gly540, Asp542, Asp503 | ||||
| Asp539, Gln642, Leu546 | |||||||
| Glu543 | |||||||
| 9-Heptadecanone | 10887 | −5.4 | Lys478 | RCOR′ | 2.80 | Asn607, Gln548, Pro398 | |
| Arg608, Ile667, Tyr419 | |||||||
| Asn421, Ser473, Pro666 | |||||||
| Glu418, Asp472, His665 | |||||||
| Val664, Gln402, Glu552 | |||||||
| Palmitic acid | 985 | −4.8 | Asn660, Lys396 | RCOR′, R-OH | 2.93, 2.95 | Val664, Pro398, Glu552 | |
| Gln662, Asp395, Leu393 | |||||||
| Leu394, Gln402, Pro397 | |||||||
| Stearic acid | 5281 | −6.2 | Lys396, Leu393 | R-OH | 3.04, 2.95 | Leu394, Gln402, Pro398 | |
| Lys478, His665, Ile667 | |||||||
| Pro666, Val664, Arg608 | |||||||
| Pro397, Asn660 | |||||||
| d-Lyxo-d-manno-nononic- 1,4-lactone | 535556 | −6.9 | Leu393, Lys396, Pro397 | RCOOR′, R-OH | 3.30, 2.73, 3.31 | Leu394, Asp395, Gln662 | |
| Gln402 | 3.23, 3.19, 2.66 | Asn660, Pro398 | |||||
| 3.08, 3.03, 3.15 | |||||||
| 3.26, 2.88 |
| Hydrogen Bond Interactions | Hydrophobic Interactions | ||||||
|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Binding Energy (kcal/mol) | Amino Acid Residue | R Group(s) Involved in Hydrogen Boding | Distance (Å) | Amino Acid Residue |
| 5KCV | MK-2206 dihydrochloride | 46930998 | −7.8 | N/A | N/A | N/A | Leu78, Leu202, Gln203 |
| Lys268, Trp80, Ala58 | |||||||
| Gln59 | |||||||
| Gsk-690693 | 16725726 | −7.4 | Tyr18, Asp274 | RNR′,RNHH | 3.18, 3.06 | Glu85, Phe161, Val83 | |
| Cys296, Gly294, Phe293 | |||||||
| Lys276, Leu316, Pro313 | |||||||
| Arg273, Leu295, Ile84 | |||||||
| Ipatasertib | 24788740 | −7.3 | N/A | N/A | N/A | Lys268, Asn53, Gln79 | |
| Ala58, Phe225, Leu223 | |||||||
| Leu202, Leu78, Trp80 | |||||||
| Capivasertib | 25227436 | −7.1 | Val83 | ROH | 2.95 | Ile84, Arg273, Asn279 | |
| Asp274, Thr291, Glu278 | |||||||
| Phe293, Gly294, Cys296 | |||||||
| Glu85, Glu17 | |||||||
| AT7867 | 11175137 | −7.6 | Ser56 | RNH | 3.04 | Lys268, Trp80, Asn53 | |
| Leu78, Cys60, Gln79 | |||||||
| Ala58, Cys77, Val201 | |||||||
| Leu202, Gln203 | |||||||
| A-674563 | 11314340 | −7.6 | Gln79, Gln203 | RNHH, RNH | 3.16, 3.20 | Ser56, Trp80, Leu202 | |
| Lys268, Asn53, Ala58 | |||||||
| Leu78 | |||||||
| Miransertib | 53262401 | −9.0 | Gln203, Leu78 | RNHH | 3.19, 3.22 | Lys268, Trp80, Leu202 | |
| Phe225, Ser216, Val201 | |||||||
| Gln59, Gln79, Ala58 | |||||||
| BAY1125976 | 70817911 | −9.1 | Asp274 | RNHH | 3.29 | Gly294, Thr291, Glu278 | |
| Asn279, Tyr229, Leu156 | |||||||
| Glu234, Phe293, Lys158 | |||||||
| Cys296 | |||||||
| Akti-1/2 | 135398501 | −8.9 | Cys296, Arg15 | RCOR′, RNR′ | 3.03, 3.15, 3.19 | Glu278, Gly294, Leu295 | |
| Val83, Gln85, Glu17 | |||||||
| Tyr18, Ile84, Phe293 | |||||||
| Asn279 | |||||||
| Uprosertib | 51042438 | −6.8 | Cys296, Leu295, Glu278 | RCOR′, RCOR′, RNHH | 2.93, 2.86, 2.93 | Tyr18, Arg273, Asp274 | |
| Lys276, Thr291, Phe293 | |||||||
| Asn279, Gly294, Ile84 | |||||||
| Thr82 | |||||||
| Afuresertib | 46843057 | −6.9 | Gly394, Gly395 | RNHH | 3.10, 2.95 | Ala50, Arg328, Lys389 | |
| Pro388, Ala329, Asp325 | |||||||
| Gly327, Tyr326, Phe55 | |||||||
| Ile36, Leu52, Pro51 | |||||||
| AT13148 | 24905401 | −6.9 | Glu341, Tyr315, His354 | ROR′, ROR′, RNR′ | 3.01, 2.70, 3.14 | Phe236, Glu278, Leu347 | |
| Pro313, Tyr350, Glu314 | |||||||
| Oridonin | 5321010 | −8.3 | Asp325, Ala50, Arg328 | RCOR′, ROH, ROH, ROH | 2.67, 2.71, 2.80, 3.24 | Phe55, Ile36, Leu52 | |
| Gly394, Ala329, Gly327 | |||||||
| Tyr326 |
| Parameters | Compound Name |
|---|---|
| Urs-12-en-24-oic acid, 3-oxo-, Methyl Ester | |
| Ames toxicity | NAT |
| Carcinogens | NC |
| Acute oral toxicity | Ⅲ |
| Rat acute toxicity | 2.1675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, K.-K.; Adnan, M.; Cho, D.-H. Network Pharmacology-Based Study to Uncover Potential Pharmacological Mechanisms of Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.) Flower against Cancer. Molecules 2021, 26, 5904. https://doi.org/10.3390/molecules26195904
Oh K-K, Adnan M, Cho D-H. Network Pharmacology-Based Study to Uncover Potential Pharmacological Mechanisms of Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.) Flower against Cancer. Molecules. 2021; 26(19):5904. https://doi.org/10.3390/molecules26195904
Chicago/Turabian StyleOh, Ki-Kwang, Md. Adnan, and Dong-Ha Cho. 2021. "Network Pharmacology-Based Study to Uncover Potential Pharmacological Mechanisms of Korean Thistle (Cirsium japonicum var. maackii (Maxim.) Matsum.) Flower against Cancer" Molecules 26, no. 19: 5904. https://doi.org/10.3390/molecules26195904