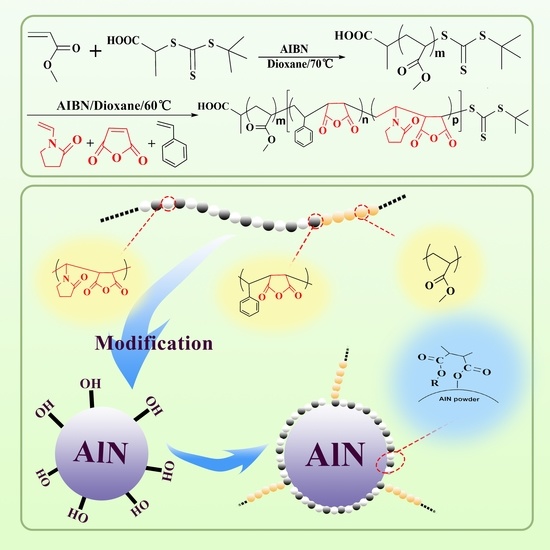
Preparation of Quaternary Amphiphilic Block Copolymer PMA-b-P (NVP/MAH/St) and Its Application in Surface Modification of Aluminum Nitride Powders
Abstract
:1. Introduction
2. Results and Discussion
2.1. Copolymer Design and Structure Analysis
2.2. FT-IR Characterization
2.3. H-NMR Characterization
2.4. AlN Powder Modified by PMA-b-P (NVP/MAH/St) Block Copolymer
2.4.1. Hydrolysis Test
2.4.2. XRD Analysis
2.4.3. SEM, TEM and EDS Analysis
3. Materials and Methods
3.1. Materials
3.2. Characterization of the Copolymer
3.3. Experimental Process
3.3.1. Synthesis of Chain Transfer Agent 2-(((tert-butyl thio)carbonothioyl)thio) Propanoic Acid (BCSPA)
3.3.2. Synthesis of Macromolecular Chain Transfer Agent PMA-CTA
3.3.3. Synthesis of Amphiphilic Block Copolymer PMA-b-P (NVP/MAH/St)
3.4. Effect Verification of AIN Powder Surface Modification
3.4.1. Modified Treatment
3.4.2. Hydrolysis Test and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids. 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Watari, K. High thermal conductivity non-oxide ceramics. J. Ceram. Soc. Jpn. 2001, 109, s7–s16. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Sugahara, Y. Pyrolytic organic-to-inorganic conversion of precursors into AIN—A review. J. Ceram. Soc. Jpn. 2006, 114, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, I.; Thiyagarajan, N.; Sundararajan, G. A non-aqueous processing route for phosphate-protection of AIN powder against hydrolysis. J. Eur. Ceram. Soc. 2008, 28, 2281–2288. [Google Scholar] [CrossRef]
- Groat, E.A.; Mroz, T.J. Aqueous slip casting of stabilized AIN powders. Am. Ceram. Soc. Bull. 1994, 73, 75–78. [Google Scholar]
- Shimizu, Y.; Hatano, J.; Hyodo, T.; Egashira, M. Ion-exchange loading of yttrium acetate as a sintering aid on aluminum nitride powder via aqueous processing. J. Am. Ceram. Soc. 2000, 83, 2793–2797. [Google Scholar] [CrossRef]
- Koh, Y.H.; Choi, J.J.; Kim, H.E. Strengthening and prevention of oxidation of aluminum nitride by formation of a silica layer on the surface. J. Am. Ceram. Soc. 2000, 83, 306–310. [Google Scholar] [CrossRef]
- Shan, H.B.; Zhu, Y.; Zhang, Z.T. Surface treatment and hydrolysis kinetics of organic films coated AlN powder. Br. Ceram. Trans. 1999, 98, 146–150. [Google Scholar] [CrossRef]
- Wang, Y. Water Soluble Polymer; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Li, B.; Huang, Y.; Pan, C. Upgrading comprehensive performances of gel polymer electrolyte based on polyacrylonitrile via copolymerizing acrylonitrile with N-vinylpryrrolidone. Electrochim. Acta 2019, 320, 134572. [Google Scholar] [CrossRef]
- Reddy, B.S.R.; Balasubramaiam, S.; Suriyan, M.R. 4-Acetamidophenyl acrylate copolymers with acrylonitrile and N-vinyl-2-pyrrolidone: Synthesis, characterization, and reactivity ratios. Appl. Polym. Sci. 2006, 99, 1919–1927. [Google Scholar] [CrossRef]
- Sambasivudu, K.; Maheedhar, G.; Srinivasa Rao, V.; Syam Kumar, M.V.; Shailaja, D. Synthesis and Amphiphilic 4-Vinyl Pyridine and n-Vinyl Pyrrolidone Copolymer Beads. Appl. Polym. Sci. 2006, 102, 192–197. [Google Scholar] [CrossRef]
- Bilalis, P.; Pitsikalis, M.; Hadjichristidis, N. Controlled nitroxide-mediated and reversible addition–fragmentation chain transfer polymerization of N-vinyl pyrrolidone: Synthesis of block copolymers with styrene and 2-vinylpyridine. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 659–665. [Google Scholar] [CrossRef]
- Ellinger, L.P. Advances in Macromolecular Chemistry; Acedemic Press: New York, NY, USA, 1968; Volume 1, p. 169. [Google Scholar]
- Yang, J.Z.; Otsu, T. Radical copolymerization of citraconic anhydride with styrene and terpolymerization of citraconic anhydride and maleic anhydride with styrene. Macromolecule 1992, 25, 102–107. [Google Scholar] [CrossRef]
- Kokubb, T.; Iwatsuki, S.; Tamashita, Y.Y. Studies on the Charge-Transfer Complex and Polymerization. XVII. The Reactivity of the Charge-Transfer Complex in Alternating Radical Copolymerization of Vinyl Ethers and Maleic Anhydride. Macromolecule 1968, 1, 482–488. [Google Scholar] [CrossRef]
- Georgiev, G.; Konstantinov, C.; Kabaivanov, V. Role of the charge-transfer complex during the copolymerization of N-vinylpyrrolidone and maleic anhydride. Macromolecule 1992, 25, 6302–6308. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecule 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition-fragmentation chain transfer (RAFT)/Xanthates (MADIX) polymerization. J. Polym. Sci. Part A 2005, 43, 5347–5393. [Google Scholar] [CrossRef]
- Güven, G.; Rzaev, Z. Complex-radical copolymerization of N-vinyl pyrrolidone with isostructural analogs of maleic anhydride. Polym Bull. 2008, 60, 741–752. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Hughes, R.J.; Nguyen, D. Ab initio emulsion polymerization by RAFT- controlled self-assembly. Macromolecule 2005, 38, 2191–2204. [Google Scholar] [CrossRef]
- Ran, R.; Yu, Y.; Tao, W. Photoinitiated RAFT polymerization in the presence of trithio carbonate. J. Appl. Polym. Sci. 2007, 105, 398–404. [Google Scholar] [CrossRef]









| Group | Monomer Feed (mmol) | Conversion | Acid Value (mg/g) | N Content/% | Copolymer Composition (mol%) | Mn (Mw/Mn) | |||
|---|---|---|---|---|---|---|---|---|---|
| BCSPA | CTC1 | CTC2 | CTC1 | CTC2 | |||||
| 1 | 1 | 40 | 0 | <5.6% | 528 | 6.60 | - | - | 5300 (1.86) |
| 2 | 1 | 30 | 10 | 49.6% | 526 | 3.43 | 52.1 | 47.9 | 3730 (1.45) |
| 3 | 1 | 24 | 16 | 78.3% | 535 | 3.37 | 50.4 | 49.6 | 6150 (1.38) |
| 4 | 1 | 20 | 20 | 95.6% | 532 | 3.32 | 49.9 | 50.1 | 7715 (1.46) |
| 5 | 1 | 16 | 24 | 98.3% | 534 | 2.71 | 40.6 | 59.4 | 7648 (1.54) |
| Conversion | 9.6% | 24.3% | 51.3% | 73.8% | 95.6% |
|---|---|---|---|---|---|
| N content/% | 2.68 | 2.98 | 3.15 | 3.35 | 3.31 |
| Acid value (mg/g) | 480 | 518 | 530 | 535 | 532 |
| Copolymer | a (mmol) | b (mmol) | Mn | Mw/Mn | N Content/% | Structure |
|---|---|---|---|---|---|---|
| A0 | 1 | 0 | 1310 | 1.17 | - | MA15 |
| A1 | 1 | 50 | 6020 | 1.49 | 2.62 | MA15-b-(CTC112-alt-CTC212) |
| A2 | 1 | 80 | 8410 | 1.36 | 2.81 | MA15-b-(CTC118-alt-CTC218) |
| A3 | 1 | 100 | 10500 | 1.57 | 2.93 | MA15-b-(CTC123-alt-CTC223) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, G.; Wang, S.; Xie, J.; Chen, Z.; Shi, Y. Preparation of Quaternary Amphiphilic Block Copolymer PMA-b-P (NVP/MAH/St) and Its Application in Surface Modification of Aluminum Nitride Powders. Molecules 2021, 26, 5884. https://doi.org/10.3390/molecules26195884
Wang Y, Zhu G, Wang S, Xie J, Chen Z, Shi Y. Preparation of Quaternary Amphiphilic Block Copolymer PMA-b-P (NVP/MAH/St) and Its Application in Surface Modification of Aluminum Nitride Powders. Molecules. 2021; 26(19):5884. https://doi.org/10.3390/molecules26195884
Chicago/Turabian StyleWang, Yu, Guangdong Zhu, Shun Wang, Jianjun Xie, Zhan Chen, and Ying Shi. 2021. "Preparation of Quaternary Amphiphilic Block Copolymer PMA-b-P (NVP/MAH/St) and Its Application in Surface Modification of Aluminum Nitride Powders" Molecules 26, no. 19: 5884. https://doi.org/10.3390/molecules26195884