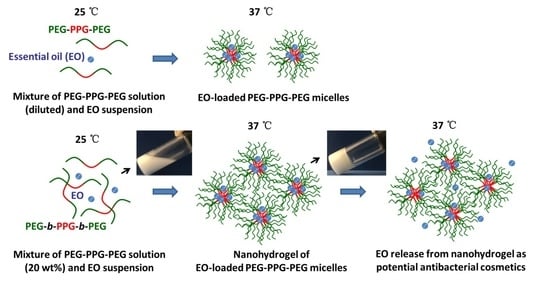
Preparation and Antibacterial Activity of Thermo-Responsive Nanohydrogels from Qiai Essential Oil and Pluronic F108
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Particle Size of QEO-Loaded PF108 Micelles
2.2. Determination of PF108 Concentration for EO Nanohydrogels by Rheological Method
2.3. Properties of EO Nanohydrogels
2.4. Determination of Encapsulation Efficiency and Loading Capacity
2.5. In Vitro Release Studies
2.6. Antibacterial Activities of QEO Nanohydrogels
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation and Characterization of QEO-Loaded PF108 Micelles
3.3. Preparation and Characterization of EO Nanohydrogels
3.4. Encapsulation Efficiency and Loading Capacity of QEO Nanohydrogels
3.5. In Vitro QEO Release from QEO Nanohydrogels
3.6. Antibacterial Activities of QEO Nanohydrogels
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant activity of Litsea glaucescens essential oil: Identification of beta-pinene and linalool as active principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef]
- Yamada, A.N.; Grespan, R.; Yamada, A.T.; Silva, E.L.; Silva-Filho, S.E.; Damiao, M.J.; De Oliveira, D.M.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Anti-inflammatory activity of Ocimum americanum L. essential oil in experimental model of zymosan-induced arthritis. Am. J. Chin. Med. 2013, 41, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Antiviral efficacy and mechanisms of action of Oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagninia, L.; Di Martino, M.C.; Lo Nostroa, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control. 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.Y.; Wang, Y.F.; Jiang, P.P.; Quek, S.Y. Antibacterial activity and mechanism of Cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, H.H.A.; Otoni, C.G.; Santana, N.A.; Oliveira, J.E. Chemical composition and antibacterial activity of Eugenia Brejoensis essential oil nanoemulsions against Pseudomonas Fluorescens. LWT-Food Sci. Technol. 2018, 93, 659–664. [Google Scholar] [CrossRef]
- Bravo, C.M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crop. Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Bañoz, S.; Gómez, Y.G. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Locatelli, M.; Navarra, M.; Ventura, C.A.; Wolfram, J.; Carafa, M.; Morittu, V.M.; Britti, D.; Marzio, L.D.; et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblaoma cells. Colloids Surf. B Biointerfaces 2013, 112, 548–553. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Dias, A.M.; Guilherme, D.S.D.; Fiorentini, M. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Martin-Piñero, M.J.; Ramirez, P.; Muñoz, J.; Alfaro, M.C. Development of rosemary essential oil nanoemulsions using a wheat biomass-derived surfactant. Colloids Surf. B Biointerfaces 2019, 173, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In situ gelling biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, T.; Duch, M.C.; Ji, Z.; Zhang, H.; Li, R.; Sun, B.; Lin, S.; Meng, H.; Liao, Y.P.; et al. Pluronic F108 coating decreases the lung fibrosis potential of multiwall carbon nanotubes by reducing lysosomal injury. Nano Lett. 2012, 12, 3050–3061. [Google Scholar] [CrossRef]
- Corey, J.M.; Gertz, C.C.; Sutton, T.J.; Chen, Q.; Mycek, K.B.; Wang, B.S.; Martin, A.A.; Johnson, S.L.; Feldman, E.L. Patterning Ntype and S-type neuroblastoma cells with Pluronic F108 and ECM proteins. J. Biomed. Mater. Res. A 2010, 93, 673–686. [Google Scholar] [PubMed] [Green Version]
- Loh, X.J.; Sng, K.B.C.; Li, J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly(ε-caprolactone), poly(ethylene glycol) and poly(propylene glycol). Biomaterials 2008, 29, 3185–3194. [Google Scholar] [CrossRef]
- Miao, T.; Fenn, S.L.; Charron, P.N.; Oldinski, R.A. Self-healing and thermoresponsive dual-cross-linked alginate hydrogels based on supramolecular inclusion complexes. Biomacromolecules 2015, 16, 3740–3750. [Google Scholar] [CrossRef] [Green Version]
- Xiang, F.; Bai, J.H.; Tan, X.B.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crop. Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Liakos, I.L.; D’autilia, F.; Garzoni, A.; Bonferoni, C.; Scarpellini, A.; Brunetti, V.; Carzino, R.; Bianchini, P.; Pompa, P.P.; Athanassiou, A. All natural cellulose acetate—Lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharmaceut. 2016, 510, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Amirmahani, N.; Mahmoodi, N.O.; Mohammadi, G.M.; Ghavidast, A. Advances in nanomicelles for sustained drug delivery. J. Ind. Eng. Chem. 2017, 55, 21–34. [Google Scholar] [CrossRef]
- Poudel, A.J.; He, F.; Huang, L.; Xiao, L.; Yang, G. Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles and α-cyclodextrin for potential injectable drug delivery system. Carbohydr. Polym. 2018, 194, 69–79. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, L.; Moingeon, F.; Gauthier, M.; Yang, G. pH-responsive poly (ethylene glycol)-block-polylactide micelles for tumor-targeted drug delivery. Biomacromolecules 2017, 18, 2711–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, A.M.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 2018, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Chabba, S.; Vashishat, R.; Mahajan, R.K. Influence of head group on the interactional behavior of cationic surface active ionic liquids with pluronic F108 in aqueous medium. J. Mol. Liq. 2016, 222, 691–702. [Google Scholar] [CrossRef]
- Morales, M.E.; Gallardo, V.; Clares, B.; García, M.B.; Ruiz, M.A. Study and description of hydrogels and organogels as vehicles for cosmetic active ingredients. J. Cosmet. Sci. 2009, 60, 627–636. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int. J. Mol. Sci. 2011, 12, 4872–4884. [Google Scholar] [CrossRef]
- De Oliveira, E.F.; Paula, H.C.B.; De Paula, R.C.M. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2018, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, characterisation and antibacterial activity of Lemon myrtle and Anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, N.; Matsen, F.A.; Zhang, M. PEG-Grafted chitosan as an injectable thermoreversible hydrogel. Macromol. Biosci. 2005, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Gündel, S.S.; De Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; Vaucher, R.A.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions containing Cymbopogon flexuosus essential oil: Development, characterization, stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Giovino, M.; Pribyl, J.; Benicewicz, B.; Kumar, S.; Schadler, L. Linear rheology of polymer nanocomposites with polymer-grafted nanoparticles. Polymer 2017, 131, 104–110. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.L.; Thangaraj, B.; Abdel-Samie, M.A.S.; Cui, H.Y. Improving the stability of thyme essential oil solid liposome by using β-cyclodextrin as a cryoprotectant. Carbohyd. Polym. 2018, 188, 243–251. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; Van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT-Food Sci. Technol. 2018, 87, 413–422. [Google Scholar] [CrossRef]
- He, F.; Li, K.; Zhang, X.H.; Yang, Y.; Fang, Y.P.; Xiang, F. Components and antibacterial activity of a novel essential oil from the nutrient broth of Eremothecium ashbyii H4565. LWT-Food Sci. Technol. 2018, 101, 389–394. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; He, F.; Chen, S.; Poudel, A.J.; Yang, Y.; Xiao, L.; Xiang, F.; Li, S. Preparation and Antibacterial Activity of Thermo-Responsive Nanohydrogels from Qiai Essential Oil and Pluronic F108. Molecules 2021, 26, 5771. https://doi.org/10.3390/molecules26195771
Zhan J, He F, Chen S, Poudel AJ, Yang Y, Xiao L, Xiang F, Li S. Preparation and Antibacterial Activity of Thermo-Responsive Nanohydrogels from Qiai Essential Oil and Pluronic F108. Molecules. 2021; 26(19):5771. https://doi.org/10.3390/molecules26195771
Chicago/Turabian StyleZhan, Jianfeng, Feng He, Shuxian Chen, Abishek Jung Poudel, Ying Yang, Lin Xiao, Fu Xiang, and Shiming Li. 2021. "Preparation and Antibacterial Activity of Thermo-Responsive Nanohydrogels from Qiai Essential Oil and Pluronic F108" Molecules 26, no. 19: 5771. https://doi.org/10.3390/molecules26195771