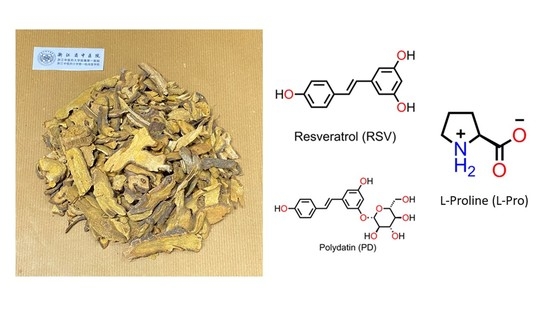
Co-Crystals of Resveratrol and Polydatin with L-Proline: Crystal Structures, Dissolution Properties, and In Vitro Cytotoxicities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterizations
2.2. Crystal Structure Analysis
2.3. Hirshfeld Surface Analysis of RSV, PD, and Their Co-Crystals with L-Pro
2.4. Solubility Analyses of RSV, RSV-L-Pro, PD, and PD-L-Pro
2.5. MTT Assays
3. Materials and Methods
3.1. General
3.2. Synthesis of Co-Crystals RSV-L-Pro and PD-L-Pro
3.3. Single-Crystal X-ray Crystallography
3.4. Solubility and Dissolution Measurement
3.5. Cell Culture
3.6. Cytotoxicity Evaluation by MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, H.L.; Gao, J.P.; Han, Y.L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Ince, S.; Arslan Acaroz, D.; Neuwirth, O.; Demirel, H.H.; Denk, B.; Kucukkurt, I.; Turkmen, R. Protective effect of polydatin, a natural precursor of resveratrol, against cisplatin-induced toxicity in rats. Food Chem. Toxicol. 2014, 72, 147–153. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Xu, J.; Zeng, Y.; Chen, L.; Xu, X.L. Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine 2019, 62, 152935. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Z.; Dong, X.; Xue, X.; Liu, Y.; Xu, S.; Zhang, J.; Han, J.; Yang, Y.; Wang, H. Polydatin Protects Diabetic Heart against Ischemia-Reperfusion Injury via Notch1/Hes1-Mediated Activation of Pten/Akt Signaling. Oxidative Med. Cell. Longev. 2018, 2018, 2750695. [Google Scholar] [CrossRef] [Green Version]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zeng, Y.N.; Zhang, K.; Zhao, Y.; Wu, Y.Y.; Li, G.; Cheng, H.Y.; Zhang, M.; Lai, F.; Wang, J.B.; et al. Polydatin Increases Radiosensitivity by Inducing Apoptosis of Stem Cells in Colorectal Cancer. Int. J. Biol. Sci. 2019, 15, 430–440. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Sun, Z.; Shen, X.; Li, L.; Jin, L.; Mao, S. Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int. J. Pharm. 2019, 569, 118562. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [Green Version]
- Murgia, D.; Mauceri, R.; Campisi, G.; De Caro, V. Advance on Resveratrol Application in Bone Regeneration: Progress and Perspectives for Use in Oral and Maxillofacial Surgery. Biomolecules 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bheereddy, P.; Yerra, V.G.; Kalvala, A.K.; Sherkhane, B.; Kumar, A. SIRT1 Activation by Polydatin Alleviates Oxidative Damage and Elevates Mitochondrial Biogenesis in Experimental Diabetic Neuropathy. Cell. Mol. Neurobiol. 2020, 41, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; El-Shahawy, A.; Yousef, A.I.; Abd El-Twab, S.M.; Elden, Z.E.; Taha, M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int. J. Biol. Macromol. 2020, 154, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Seljak, K.B.; Berginc, K.; Trontelj, J.; Zvonar, A.; Kristl, A.; Gasperlin, M. A self-microemulsifying drug delivery system to overcome intestinal resveratrol toxicity and presystemic metabolism. J. Pharm. Sci. 2014, 103, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Spogli, R.; Bastianini, M.; Ragonese, F.; Iannitti, R.G.; Monarca, L.; Bastioli, F.; Nakashidze, I.; Brecchia, G.; Menchetti, L.; Codini, M.; et al. Solid Dispersion of Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Microparticles Improves Oral Bioavailability. Nutrients 2018, 10, 1925. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, Y.; Xiao, H.; Liu, Y.; Mo, H.; Ma, H.; Liang, G. Characterization of the Supermolecular Structure of Polydatin/6-O-alpha-Maltosyl-beta-cyclodextrin Inclusion Complex. J. Food Sci. 2015, 80, C1156–C1161. [Google Scholar] [CrossRef]
- Lin, L.; Gong, H.; Li, R.; Huang, J.; Cai, M.; Lan, T.; Huang, W.; Guo, Y.; Zhou, Z.; An, Y.; et al. Nanodrug with ROS and pH Dual-Sensitivity Ameliorates Liver Fibrosis via Multicellular Regulation. Adv. Sci. Suppleme 2020, 7, 1903138. [Google Scholar] [CrossRef]
- An, S.; He, J.; Sun, L.; Ren, D.; Ban, Y. Investigation of the inclusion behavior of HP–β-cyclodextrin with polydatin in solution and its analytical application. J. Mol. Struct. 2013, 1037, 9–14. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Yadav, B.; Shastri, N.R. Continuous manufacturing of co-crystals: Challenges and prospects. Drug Deliv. Transl. Res. 2018, 8, 1726–1739. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Raheem Thayyil, A.; Juturu, T.; Nayak, S.; Kamath, S. Pharmaceutical Co-Crystallization: Regulatory Aspects, Design, Characterization, and Applications. Adv. Pharm. Bull. 2020, 10, 203–212. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, Q.; Li, M.; Wang, J.-R.; Mei, X. Modulating the Dissolution and Mechanical Properties of Resveratrol by Cocrystallization. Cryst. Growth Des. 2017, 17, 3989–3996. [Google Scholar] [CrossRef]
- Gong, N.; Wang, X.; Wang, Y.; Yang, S.; Song, J.; Lu, Y.; Du, G. Control over Polymorph Formation of Polydatin in Binary Solvent System and Structural Characterization. J. Pharm. Biomed. Anal. 2020, 190, 113260. [Google Scholar] [CrossRef]
- Bonilla-Landa, I.; Cuapio-Munoz, U.; Luna-Hernandez, A.; Reyes-Luna, A.; Rodriguez-Hernandez, A.; Ibarra-Juarez, A.; Suarez-Mendez, G.; Barrera-Mendez, F.; Caram-Salas, N.; Enriquez-Medrano, J.F.; et al. l-Proline as a Valuable Scaffold for the Synthesis of Novel Enantiopure Neonicotinoids Analogs. J. Agric. Food Chem. 2021, 69, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Komara, S.W.; Horikawa, A.; Uekusa, H. Composing Novel Diclofenac Potassium and l-Proline Salt Cocrystal as a Strategy to Increase Solubility and Dissolution. J. Pharm. Sci. 2020, 109, 3423–3438. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, S.; Zhao, X.; Zhou, S. l-Proline protects mice challenged by Klebsiella pneumoniae bacteremia. J. Microbiol. Immunol. Infect. 2021, 54, 213–220. [Google Scholar] [CrossRef]
- Liu, M.; Hong, C.; Yao, Y.; Shen, H.; Ji, G.; Li, G.; Xie, Y. Development of a pharmaceutical cocrystal with solution crystallization technology: Preparation, characterization, and evaluation of myricetin-proline cocrystals. Eur. J. Pharm. Biopharm. 2016, 107, 151–159. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.-Y.; Liu, F.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. Simultaneously enhancing the in vitro/in vivo performances of acetazolamide using proline as a zwitterionic coformer for cocrystallization. CrystEngComm 2019, 21, 3064–3073. [Google Scholar] [CrossRef]
- Inam, M.; Liu, L.; Wang, J.W.; Yu, K.X.; Phan, C.U.; Shen, J.; Zhang, W.H.; Tang, G.; Hu, X. Enhancing the Physiochemical Properties of Puerarin via L-Proline Co-Crystallization: Synthesis, Characterization, and Dissolution Studies of Two Phases of Pharmaceutical Co-Crystals. Int. J. Mol. Sci. 2021, 22, 928. [Google Scholar] [CrossRef] [PubMed]
- Parwani, A.V. Expression of Glypican 3 in Ovarian and Extragonadal Germ Cell Tumors. Yearb. Pathol. Lab. Med. 2009, 2009, 93–95. [Google Scholar] [CrossRef]
- Zou, J.; Yang, Y.; Yang, Y.; Liu, X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-kappaB pathway. Biomed. Pharm. 2018, 108, 130–136. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Zhu, L.Y.; Yang, Y.Q.; Zhang, Z.H.; Chen, Q.G.; Sun, Y.P.; Bi, J.J.; Luo, X.M.; Ni, Z.H.; Wang, X.B. Resveratrol inhibits MUC5AC expression by regulating SPDEF in lung cancer cells. Phytomedicine 2021, 89, 153601. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT and SADABS; Bruker ACS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Farrugia, L.J. WinGX Suite For Smallmolecule Single-crystal Crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]










| Crystal Data | RSV | RSV-L-Pro | PD | PD-L-Pro |
|---|---|---|---|---|
| CCDC No. | 2090218 | 2090219 | 2090216 | 2090217 |
| Formula | C14H12O3 | C14H12O3·2(C5H9NO2) | C20H22O8 | C20H22O8·3(C5H9NO2)·C2H6O |
| M.W. | 228.24 | 458.50 | 390.37 | 781.84 |
| Crystal system | Monoclinic | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | P21/c | P212121 | P212121 | C2 |
| Temperature (K) | 170(2) | 170(2) | 170(2) | 170(2) |
| a (Å) | 4.3472(2) | 5.55850(10) | 7.3126(14) | 39.40(3) |
| b (Å) | 9.2128(4) | 18.6566(4) | 7.6691(14) | 5.232(3) |
| c (Å) | 26.6588(10) | 21.5392(5) | 33.051(5) | 25.96(2) |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 92.953(2) | 90 | 90 | 128.34(3) |
| γ (°) | 90 | 90 | 90 | 90 |
| V (Å3) | 1066.26(8) | 2233.67(8) | 1853.5(6) | 4198(6) |
| Z | 4 | 4 | 4 | 4 |
| Dc/(g cm−3) | 1.422 | 1.363 | 1.399 | 1.240 |
| F(000) | 480 | 976 | 824 | 1680 |
| μ (mm−1) | 0.100 | 0.833 | 0.916 | 0.804 |
| Measured reflections | 13,844 | 16,590 | 15,646 | 26,042 |
| Independent reflections | 1998 | 4001 | 3344 | 6730 |
| Observed reflections | 2359 | 4078 | 3386 | 7392 |
| Flack parameter | / | 0.05(7) | 0.06(4) | −0.03(8) |
| Rint | 0.0397 | 0.0386 | 0.0400 | 0.0628 |
| R (F2 > 2σ(F2)) | 0.0441 | 0.0413 | 0.0280 | 0.0584 |
| wR(F2) | 0.1124 | 0.1088 | 0.0725 | 0.1680 |
| GOF | 1.058 | 1.043 | 1.072 | 1.091 |
| Parameters | 154 | 301 | 259 | 508 |
| Δρmax, Δρmin (e Å−3) | 0.254, −0.373 | 0.674, −0.261 | 0.166, −0.238 | 0.849, −0.412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, Y.; Yu, K.; Wu, X.; Wang, Z.; Cui, Y.; Bao, H.; Wang, J.; Hu, X.; Ji, Y.; Tang, G. Co-Crystals of Resveratrol and Polydatin with L-Proline: Crystal Structures, Dissolution Properties, and In Vitro Cytotoxicities. Molecules 2021, 26, 5722. https://doi.org/10.3390/molecules26185722
Lou Y, Yu K, Wu X, Wang Z, Cui Y, Bao H, Wang J, Hu X, Ji Y, Tang G. Co-Crystals of Resveratrol and Polydatin with L-Proline: Crystal Structures, Dissolution Properties, and In Vitro Cytotoxicities. Molecules. 2021; 26(18):5722. https://doi.org/10.3390/molecules26185722
Chicago/Turabian StyleLou, Yijie, Kaxi Yu, Xiajun Wu, Zhaojun Wang, Yusheng Cui, Hanxiao Bao, Jianwei Wang, Xiurong Hu, Yunxi Ji, and Guping Tang. 2021. "Co-Crystals of Resveratrol and Polydatin with L-Proline: Crystal Structures, Dissolution Properties, and In Vitro Cytotoxicities" Molecules 26, no. 18: 5722. https://doi.org/10.3390/molecules26185722