Nitropyridines as 2π-Partners in 1,3-Dipolar Cycloadditions with N-Methyl Azomethine Ylide: An Easy Access to Condensed Pyrrolines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Starting 2-Substitutied 5-R-3nitropyridines 2a–q
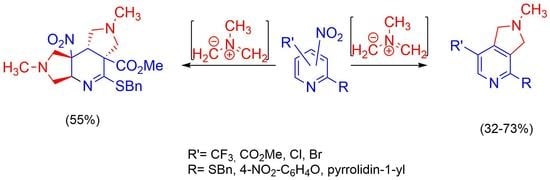
2.2. [3+2]-Cycloaddition of Nitropyridines 2
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Compounds 2a, 2d, 2g, 2h, 2k, 2l, 2o
3.3. Synthesis of Compounds 2b, 2e, 2i, 2m, 2p
3.4. Synthesis of Compounds 2c, 2f, 2j, 2n, 2q
3.5. Synthesis of Compounds 4a–j, 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medical Importnace of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- McAteer, C.H.; Balasubramanian, M.; Murugan, R. Comprehensive Heterocyclic Chemistry III; Karitzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Jones, G., Eds.; Elsevier: New York, NY, USA, 2008; Volume 7, p. 309. [Google Scholar]
- Khan, E. Pyridine Derivatives as Biologically Active Precursors; Organics and Selected Coordination Complexes. ChemistrySelect 2021, 6, 3041–3064. [Google Scholar] [CrossRef]
- Jarman, M.; Barrie, A.S.E.; Llera†, J.M. The 16,17-Double Bond Is Needed for Irreversible Inhibition of Human Cytochrome P45017α by Abiraterone (17-(3-Pyridyl)androsta-5,16-dien-3β-ol) and Related Steroidal Inhibitors. J. Med. Chem. 1998, 41, 5375–5381. [Google Scholar] [CrossRef] [PubMed]
- Guarna, A.; Occhiato, E.G.; Machetti, F.; Giacomelli, V. 19-Nor-10-azasteroids. 5.1A Synthetic Strategy for the Preparation of (+)-17-(3-Pyridyl)-(5β)-10-azaestra-1,16-dien-3-one, a Novel Potential Inhibitor for Human Cytochrome P45017α(17α-Hydroxylase/C17,20-lyase). J. Org. Chem. 1999, 64, 4985–4989. [Google Scholar] [CrossRef] [PubMed]
- Paget, S.D.; Foleno, B.D.; Boggs, C.M.; Goldschmidt, R.M.; Hlasta, D.J.; Weidner-Wells, M.A.; Werblood, H.M.; Wira, E.; Bush, K.; Macielag, M.J. Synthesis and antibacterial activity of pyrroloaryl-substituted oxazolidinones. Bioorg. Med. Chem. Lett. 2003, 13, 4173–4177. [Google Scholar] [CrossRef] [PubMed]
- Catalano, J.G.; Gudmundsson, K.S.; Svolto, A.; Boggs, S.D.; Miller, J.F.; Spaltenstein, A.; Thomson, M.; Wheelan, P.; Minick, D.J.; Phelps, D.P.; et al. Synthesis of a novel tricyclic 1,2,3,4,4a,5,6,10b-octahydro-1,10-phenanthroline ring system and CXCR4 antagonists with potent activity against HIV-1. Bioorg. Med. Chem. Lett. 2010, 20, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.B.; Tamayo, N.A.; Bartberger, M.D.; Bo, Y.; Clarine, J.; Davis, C.D.; Gore, V.K.; Kaller, M.R.; Lehto, S.G.; Ma, V.V.; et al. Optimization of Potency and Pharmacokinetic Properties of Tetrahydroisoquinoline Transient Receptor Potential Melastatin 8 (TRPM8) Antagonists. J. Med. Chem. 2014, 57, 2989–3004. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Ito, N.; Souzo, I.; Isomura, Y.; Homma, H.; Murakami, M. Nitrogen-containing heterocyclic ring derivatives. U.S. Patent 4284778 (A), 18 August 1981. [Google Scholar]
- Koeckritz, P.; Liebscher, J.; Huebler, D. Verfahren zur herstellung von 1.2.4-Triazolo/1.5-a/-pyridin-8-carbonitrilen. Ger. Patent DD280110 (A1), 27 June 1990. [Google Scholar]
- Guba, W.; Nettekoven, M.; Püllmann, B.; Riemer, C.; Schmitt, S. Comparison of inhibitory activity of isomeric triazolopyridine derivatives towards adenosine receptor subtypes or do similar structures reveal similar bioactivities? Bioorg. Med. Chem. Lett. 2004, 14, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Selby, T.P. Substituted phenyltriazolopyrimidine herbicides. WO Patent 9012012, 18 October 1990. [Google Scholar]
- Bertuzzi, G.; Bernardi, L.; Fochi, M. Nucleophilic Dearomatization of Activated Pyridines. Catalysts 2018, 8, 632. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, G.; Sathiyanarayanan, K. Dearomatization Strategies of Heteroaromatic Compounds. Curr. Organocatal. 2015, 2, 14–26. [Google Scholar] [CrossRef]
- Dudnik, A.S.; Weidner, V.L.; Motta, A.; Delferro, M.; Marks, T.J. Atom-efficient regioselective 1, 2-dearomatization of functionalized pyridines by an earth-abundant organolanthanide catalyst. Nat. Chem. 2014, 6, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.P.; Porco, J.A. Dearomatization Strategies in the Synthesis of Complex Natural Products. Angew. Chem. Int. Ed. 2011, 50, 4068–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.L.; Chordia, M.; Harman, W.D. Synthetic applications of the dearomatization agent pentaammineosmium(II). Tetrahedron 2001, 57, 8203–8225. [Google Scholar] [CrossRef]
- Wertjes, W.C.; Southgate, E.H.; Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 2018, 47, 7996–8017. [Google Scholar] [CrossRef] [PubMed]
- Bastrakov, M.A.; Kucherova, A.Y.; Fedorenko, A.K.; Starosotnikov, A.M.; Fedyanin, I.V.; Dalinger, I.L.; Shevelev, S.A. Dearomatization of 3,5-dinitropyridines–an atom-efficient approach to fused 3-nitropyrrolidines. Arkivoc 2017, 2017, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Bastrakov, M.A.; Fedorenko, A.K.; Starosotnikov, A.M.; Kachala, V.V.; Shevelev, S.A. Dearomative (3+2) cycloaddition of 2-substituted 3,5-dinitropyridines and N-methyl azomethine ylide. Chem. Heterocycl. Compd. 2019, 55, 72–77. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Starosotnikov, A.M.; Pechenkin, S.Y.; Kachala, V.V.; Glukhov, I.V.; Shevelev, S.A. Double 1,3-dipolar cycloaddition of N-methyl Azomethine ylide to meta-Dinitrobenzene annelated with nitrogen aromatic heterocycles. J. Heterocycl. Chem. 2010, 47, 893–896. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bastrakov, M.A.; Starosotnikov, A.M.; Glukhov, I.V.; Lysov, K.A.; Rakitin, O.A.; Shevelev, S.A. 4,6-Dinitrobenzo[c]isothiazole: Synthesis and 1,3-dipolar cycloaddition to azomethine ylide. Mendeleev Commun. 2010, 20, 353–354. [Google Scholar] [CrossRef]
- Lee, S.; Diab, S.; Queval, P.; Sebban, M.; Chataigner, I.; Piettre, S.R. Aromatic C=C Bonds as Dipolarophiles: Facile Reactions of Uncomplexed Electron-Deficient Benzene Derivatives and Other Aromatic Rings with a Non-Stabilized Azomethine Ylide. Chem. Eur. J. 2013, 19, 7181–7192. [Google Scholar] [CrossRef] [PubMed]





| Compound | R3H | R1 | R2 | Conditions * | Yield (%) |
|---|---|---|---|---|---|
| 2a | BnSH | CF3 | NO2 | A | 88 |
| 2b | 4-NO2-C6H4OH | CF3 | NO2 | B | 79 |
| 2c |  | CF3 | NO2 | C | 95 |
| 2d | BnSH | CO2Me | NO2 | A | 95 |
| 2e | 4-NO2-C6H4OH | CO2Me | NO2 | B | 93 |
| 2f |  | CO2Me | NO2 | C | 92 |
| 2g | i-BuSH | CO2Me | NO2 | A | 85 |
| 2h | BnSH | Cl | NO2 | A | 77 |
| 2i | 4-NO2-C6H4OH | Cl | NO2 | B | 81 |
| 2j |  | Cl | NO2 | C | 91 |
| 2k | BnSH | Me | NO2 | A | 58 |
| 2l | BnSH | Br | NO2 | A | 68 |
| 2m | 4-NO2-C6H4OH | Br | NO2 | B | 74 |
| 2n |  | Br | NO2 | C | 86 |
| 2o | BnSH | NO2 | CO2Me | A | 98 |
| 2p | 4-NO2-C6H4OH | NO2 | CO2Me | B | 92 |
| 2q |  | NO2 | CO2Me | C | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastrakov, M.A.; Fedorenko, A.K.; Starosotnikov, A.M.; Shakhnes, A.K. Nitropyridines as 2π-Partners in 1,3-Dipolar Cycloadditions with N-Methyl Azomethine Ylide: An Easy Access to Condensed Pyrrolines. Molecules 2021, 26, 5547. https://doi.org/10.3390/molecules26185547
Bastrakov MA, Fedorenko AK, Starosotnikov AM, Shakhnes AK. Nitropyridines as 2π-Partners in 1,3-Dipolar Cycloadditions with N-Methyl Azomethine Ylide: An Easy Access to Condensed Pyrrolines. Molecules. 2021; 26(18):5547. https://doi.org/10.3390/molecules26185547
Chicago/Turabian StyleBastrakov, Maxim A., Alexey K. Fedorenko, Alexey M. Starosotnikov, and Alexander Kh. Shakhnes. 2021. "Nitropyridines as 2π-Partners in 1,3-Dipolar Cycloadditions with N-Methyl Azomethine Ylide: An Easy Access to Condensed Pyrrolines" Molecules 26, no. 18: 5547. https://doi.org/10.3390/molecules26185547