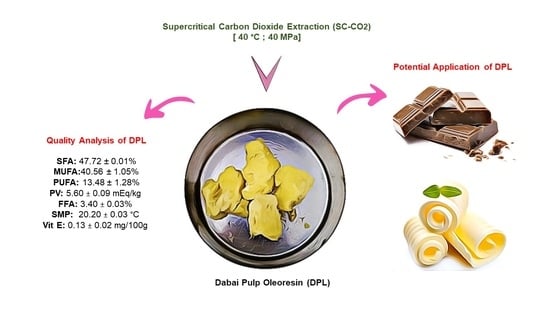
Preliminary Evaluation of Supercritical Carbon Dioxide Extracted Dabai Pulp Oleoresin as a New Alternative Fat
Abstract
:1. Introduction
2. Results and Discussion
Quality Parameters of DPL
3. Materials and Methods
3.1. SC-CO2 Extraction of DPL
3.2. Quality Analysis of DPL
3.2.1. Determination of FAC
3.2.2. Determination of PV and FFA
3.2.3. Determination of SMP
3.2.4. HPLC-FLD Analysis of Vitamin E as α-Tocopherol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Azlan, A.; Prasad, K.N.; Khoo, H.E.; Abdul-Aziz, N.; Mohamad, A.; Ismail, A.; Amom, Z. Comparison of Fatty Acids, Vitamin E and Physicochemical Properties of Canarium Odontophyllum Miq. (Dabai), Olive and Palm Oils. J. Food Compos. Anal. 2010, 23, 772–776. [Google Scholar] [CrossRef]
- Azrina, A.; Nurul Nadiah, M.N.; Amin, I. Antioxidant Properties of Methanolic Extract of Canarium Odontophyllum Fruit. Int. Food Res. J. 2010, 17, 319–326. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Ismail, A.; Abas, F. Influence of Different Extraction Media on Phenolic Contents and Antioxidant Capacity of Defatted Dabai (Canarium Odontophyllum) Fruit. Food Anal. Methods 2012, 5, 339–350. [Google Scholar] [CrossRef]
- Voon, B.H.; Kueh, H.S. The Nutritional Value of Indigenous Fruits and Vegetables in Sarawak. Asia Pac. Clin. Nutr. 1999, 8, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Beneficial Effect of Supercritical Carbon Dioxide Extracted (SC-CO2) Dabai (Canarium Odontophyllum) Pulp Oil in Hypercholesterolemia-Induced SPF Sprague-Dawley Rats. Nat. Prod. Commun. 2018, 13, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Quality of Dabai Pulp Oil Extracted by Supercritical Carbon Dioxide and Supplementation in Hypercholesterolemic Rat—A New Alternative Fat. Foods 2021, 10, 262. [Google Scholar] [CrossRef]
- Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Hepatoprotective Effect of Supercritical Carbon Dioxide Extracted Dabai Pulp Oil and Its Defatted Pulp. Molecules 2021, 26, 671. [Google Scholar] [CrossRef]
- Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Effect of Defatted Dabai Pulp Extract in Urine Metabolomics of Hypercholesterolemic Rats. Nutrients 2020, 12, 3511. [Google Scholar] [CrossRef]
- Sovilj, M.N. Critical Review of Supercritical Carbon Dioxide Extraction of Selected Oil Seeds. Acta Period. Technol. 2010, 41, 105–120. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical Fluid Extraction: Recent Advances and Applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Jiménez-Fernández, M.; Azuara, E. Oleoresins from Capsicum Spp.: Extraction Methods and Bioactivity. Food Bioprocess Technol. 2017, 10, 51–76. [Google Scholar] [CrossRef]
- Urbančič, S.; Kolar, M.H.; Dimitrijević, D.; Demšar, L.; Vidrih, R. Stabilisation of Sunflower Oil and Reduction of Acrylamide Formation of Potato with Rosemary Extract during Deep-Fat Frying. LWT Food Sci. Technol. 2014, 57, 671–678. [Google Scholar] [CrossRef]
- Honda, M.; Higashiura, T.; Fukaya, T. Safety Assessment of a Natural Tomato Oleoresin Containing High Amounts of Z-Isomers of Lycopene Prepared with Supercritical Carbon Dioxide. J. Sci. Food Agric. 2017, 97, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative Stability of Refined Olive and Sunflower Oils Supplemented with Lycopene-Rich Oleoresin from Tomato Peels Industrial by-Product, during Accelerated Shelf-Life Storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- López-Padilla, A.; Ruiz-Rodriguez, A.; Reglero, G.; Fornari, T. Supercritical Carbon Dioxide Extraction of Calendula Officinalis: Kinetic Modeling and Scaling up Study. J. Supercrit. Fluids 2017, 130, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, P.; Khanam, S. Influence of Operating Parameters on Supercritical Fluid Extraction of Essential Oil from Turmeric Root. J. Clean. Prod. 2018, 188, 816–824. [Google Scholar] [CrossRef]
- Azlan, A.; Eng, H.; Khairunisa, W.; Shapie, W.; Kadir, N.A.A. Nutritional Quality and Sensory Evaluation of Dabai-Fortified Cocoa Bar. Int. J. Food Prop. 2020, 23, 1324–1336. [Google Scholar] [CrossRef]
- Malaysia Department of Standards. MS 814:2007 Palm Oil—Specification (Second Revision); SIRIM Berhad: Shah Alam, Malaysia, 2007; Volume 814. [Google Scholar]
- Berger, K.G. Food Uses of Palm Oil; MPOPC Palm Oil Information Series: Kuala Lumpur, Malaysia, 1996; p. 25. [Google Scholar]
- Hassan, M.; Rahman, N.N.A.; Ibrahim, M.; Omar, A.M. Simple Fractionation through the Supercritical Carbon Dioxide Extraction of Palm Kernel Oil. Sep. Purif. Technol. 2000, 19, 113–120. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Nik Norulaini, N.A.; Mohd Omar, A.K.; Smith, R.L. Supercritical Carbon Dioxide (SC-CO2) Extraction of Palm Kernel Oil from Palm Kernel. J. Food Eng. 2007, 79, 1007–1014. [Google Scholar] [CrossRef]
- Akanda, M.J.H.; Sarker, M.Z.I.; Ferdosh, S.; Manap, M.Y.A.; Rahman, N.N.N.A.; Kadir, M.O.A. Applications of Supercritical Fluid Extraction (S.F.E.) of Palm Oil and Oil from Natural Sources. Molecules 2012, 17, 1764–1794. [Google Scholar] [CrossRef] [Green Version]
- Fattori, M.; Bulley, N.R.; Meisen, A. Fatty Acid and Phosphorus Contents of Canola Seed Extracts Obtained with Supercritical Carbon Dioxide. J. Agric. Food Chem. 1987, 35, 739–743. [Google Scholar] [CrossRef]
- Fatouh, A.E.; Mahran, G.A.; El-Ghandour, M.A.; Singh, R.K. Fractionation of Buffalo Butter Oil by Supercritical Carbon Dioxide. LWT Food Sci. Technol. 2007, 40, 1687–1693. [Google Scholar] [CrossRef]
- Bharath, R.; Yamane, S.; Inomata, H.; Adschiri, T.; Arai, K. Phase Equilibria of Supercritical CO2—Fatty Oil Component Binary Systems. Fluid Phase Equilib. 1993, 83, 183–192. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Sherazi, S.T.H.; Khaskheli, A.R.; Kandhro, A.A.; Uddin, S. Analytical Approaches for the Assessment of Free Fatty Acids in Oils and Fats. Anal. Methods 2014, 6, 4956–4963. [Google Scholar] [CrossRef]
- Ekwenye, U. Chemical Characteristics of Palm Oil Biodeterioration. Biokemistri 2010, 18, 141–149. [Google Scholar] [CrossRef]
- Lau, H.L.N.; Choo, Y.M.; Ma, A.N.; Chuah, C.H. Characterization and Supercritical Carbon Dioxide Extraction of Palm Oil (Elaeis Guineensis). J. Food Lipids 2006, 13, 210–221. [Google Scholar] [CrossRef]
- Naz, S.; Shabbir, M.A.; Khan, M.R.; Shahid, M. Comparison of Flaxseed Oil Characteristics of Three Pakistani Varieties Obtained by Supercritical CO2 and Two Conventional Extraction Methods. Int. Food Res. J. 2019, 26, 1599–1607. [Google Scholar]
- Kong, F.; Singh, R.P. Advances in Instrumental Methods to Determine Food Quality Deterioration. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2011; pp. 381–404. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Khalid, N. Quality Evaluation and Safety Assessment of Different Cooking Oils Available in Pakistan. J. Chem. Soc. Pak. 2012, 34, 518–525. [Google Scholar]
- Okonkwo, E.U.; Arowora, K.A.; Ogundele, B.A.; Omodara, M.A.; Afolayan, S.S. Storability and Quality Indices of Palm Oil in Different Packaging Containers in Nigeria. J. Stored Prod. Postharvest Res. 2012, 3, 177–179. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłuzewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Zbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [Green Version]
- Kanagaratnam, S.; Sahri, M.M.; Idris, N.A.; Tangavelu, T.; Ahmad, M.J. Palm-Based Trans-Free Roll-in Margarine. Palm. Oil. Dev. 1995, 48, 7–12. [Google Scholar]
- Maslina, S.; Alwi, M.; Ming, L.O. The Physicochemical Properties of Cocoa Butter Equivalent Produced From Lipase-Catalyzed Palm Oil and Hydrogenated Palm Oil via Physical Fractionation. Pertanika J. Sci. Technol. 2019, 27, 331–342. [Google Scholar]
- Sellami, M.; Ghamgui, H.; Frikha, F.; Gargouri, Y.; Miled, N. Enzymatic Transesterification of Palm Stearin and Olein Blends to Produce Zero-Trans Margarine Fat. BMC Biotechnol. 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norizzah, A.R.; Norsyamimi, M.; Zaliha, O.; Nur Azimah, K.; Siti Hazirah, M.F. Physicochemical Properties of Palm Oil and Palm Kernel Oil Blend Fractions after Interesterification. Int. Food Res. J. 2015, 22, 1390–1395. [Google Scholar]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and Total Phenolics in 10 Different Nut Types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Malekbala, M.R.; Soltani, S.M.; Hosseini, S.; Eghbali Babadi, F.; Malekbala, R. Current Technologies in the Extraction, Enrichment and Analytical Detection of Tocopherols and Tocotrienols: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2935–2942. [Google Scholar] [CrossRef]
- Gustinelli, G.; Eliasson, L.; Svelander, C.; Andlid, T.; Lundin, L.; Ahrn, L.; Alminger, M. Supercritical Fluid Extraction of Berry Seeds: Chemical Composition and Antioxidant Activity. J. Food Qual. 2018, 2018, 6046074. [Google Scholar] [CrossRef]
- Atkinson, J.; Epand, R.F.; Epand, R.M. Tocopherols and Tocotrienols in Membranes: A Critical Review. Free Radic. Biol. Med. 2008, 44, 739–764. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Chen, Y.; Kong, X.; Hua, Y. Effects of P.H. on Protein Components of Extracted Oil Bodies from Diverse Plant Seeds and Endogenous Protease-Induced Oleosin Hydrolysis. Food Chem. 2016, 200, 125–133. [Google Scholar] [CrossRef]
- IUPAC. Method 2.301: Preparation of the Fatty Acid Methyl Esters. In Standard Methods for the Analysis of Oils, Fats and Derivatives; Paquot, C., Ed.; A. Wheaton & Co. Ltd.: Durham, NC, USA, 1987; pp. 96–102. [Google Scholar]
- AOAC. Peroxide Value of Oils and Fats Method 965.33. In Official Methods of Analysis of A.O.A.C. International; Cunniff, P., Ed.; Association of Official Analytical Chemists, Inc: Washington, DC, USA, 1997; p. 12. [Google Scholar]
- Kuntom, A.M.P.O.B. Test Methods: A Compendium of Test[s] on Palm Oil Products, Palm Kernel Products, Fatty Acids, Food Related Products and Others; Malaysian Palm Oil Board, Ministry of Plantation Industries and Commodities: Bandar Baru Bangi, Selangor, 2005.
- AOAC. A.O.A.C. Official Method 971.30 a-Tocopherol and a-Tocopheryl Acetate in Foods and Feeds. In A.O.A.C. Official Method; Horwitz, W., Latimer, G., Eds.; Association of Official Analytical Chemists, Inc.: Washington, DC, USA, 2006; Volume 4, pp. 4–7. [Google Scholar]


| Fatty Acids | % 1 | |
|---|---|---|
| C8 | caprylic | 0.05 ± 0.00 |
| C10 | capric | 0.01 ± 0.00 |
| C11 | undecanoic | 0.02 ± 0.01 |
| C12 | lauric | 0.77 ± 0.00 |
| C14 | myristic | 0.28 ± 0.00 |
| C15 | pentadecanoic | 0.04 ± 0.01 |
| C16 | palmitic | 41.62 ± 0.01 |
| C17 | heptadecanoic | 0.11 ± 0.01 |
| C18 | stearic | 4.31 ± 0.00 |
| C20 | arachidic | 0.10 ± 0.00 |
| C21 | henicosanoic | 0.03 ± 0.00 |
| C22 | behenic | 0.2 ± 0.00 |
| C23 | tricosanoic | 0.11 ± 0.00 |
| C24 | lignoceric | 0.09 ± 0.00 |
| Saturated fatty acids | 47.72 ± 0.01 | |
| C15:1 | cis-10-pentadecenoic | 0.04 ± 0.01 |
| C16:1 | palmitoleic | 0.63 ± 0.02 |
| C17:1 | cis-10-heptadecanoic | 0.03 ± 0.00 |
| C18:1n9c | oleic | 39.68 ± 1.21 |
| C20:1n9 | cis-11-eicosenoic | 0.07 ± 0.00 |
| C22:1n9 | erucic | 0.02 ± 0.01 |
| C24:1 | nervonic | 0.11 ± 0.13 |
| Monounsaturated fatty acids | 40.56 ± 1.05 | |
| c18:2n6c | linoleic (cis) | 12.85 ± 1.25 |
| C18:3n6 | Υ-linolenic | 0.13 ± 0.02 |
| C18:3n3 | a-linolenic | 0.51 ± 0.01 |
| Polyunsaturated fatty acids | 13.48 ± 1.28 | |
| Quality Analysis | |
|---|---|
| Peroxide value (mEq/kg) | 5.60 ± 0.09 |
| Free fatty acid (%, as in oleic acid) | 3.40 ± 0.03 |
| Slip melting point (°C) | 20.20 ± 0.03 |
| Vitamin E (α-tocopherol) (mg/100 g) | 0.13 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadir, N.A.A.A.; Azlan, A.; Abas, F.; Ismail, I.S. Preliminary Evaluation of Supercritical Carbon Dioxide Extracted Dabai Pulp Oleoresin as a New Alternative Fat. Molecules 2021, 26, 5545. https://doi.org/10.3390/molecules26185545
Kadir NAAA, Azlan A, Abas F, Ismail IS. Preliminary Evaluation of Supercritical Carbon Dioxide Extracted Dabai Pulp Oleoresin as a New Alternative Fat. Molecules. 2021; 26(18):5545. https://doi.org/10.3390/molecules26185545
Chicago/Turabian StyleKadir, Noor Atiqah Aizan Abdul, Azrina Azlan, Faridah Abas, and Intan Safinar Ismail. 2021. "Preliminary Evaluation of Supercritical Carbon Dioxide Extracted Dabai Pulp Oleoresin as a New Alternative Fat" Molecules 26, no. 18: 5545. https://doi.org/10.3390/molecules26185545