Antispasmodic Effect of Asperidine B, a Pyrrolidine Derivative, through Inhibition of L-Type Ca2+ Channel in Rat Ileal Smooth Muscle
Abstract
:1. Introduction
2. Results
2.1. The Effect of Asperidine B on the Rat Ileal Contractile Response Induced by KCl
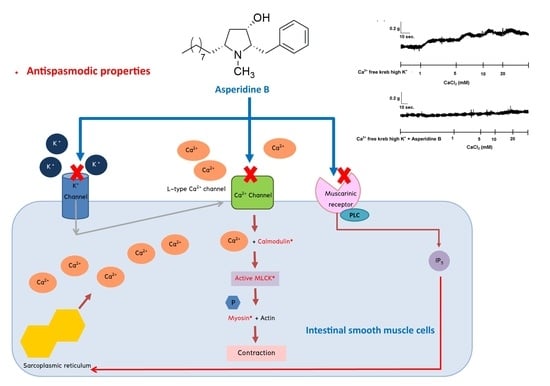
2.2. Mechanism of Asperidine B Action on Ileal Smooth Muscle Contraction
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Pyrrolidine Derivative Asperidine B Material
4.3. Animals and Tissue Preparation
4.4. Experimental Protocol
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Martínez Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural antispasmodics: Source, stereochemical configuration, and biological activity. Biomed. Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef] [Green Version]
- Lacy, B.E.; Weiser, K. Gastrointestinal motility disorders: An update. Dig. Dis. 2006, 24, 228–242. [Google Scholar] [CrossRef]
- Sanders, K.M. Regulation of smooth muscle excitation and contraction. Neurogastroenterol. Motil. 2008, 20, 39–53. [Google Scholar] [CrossRef]
- Tanaka, Y.; Horinouchi, T.; Koike, K. New insights into beta-adrenoceptors in smooth muscle: Distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin. Exp. Pharmacol. Physiol. 2005, 32, 503–514. [Google Scholar] [CrossRef]
- Ogura, T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J. Neurophysiol. 2002, 87, 2643–2649. [Google Scholar] [CrossRef] [Green Version]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta 1999, 1411, 334–350. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Saify, Z.; Gilani, A.; Iqbal, Z. Studies on the effects of piperidine derivatives on blood pressure and smooth muscles contractions. Arch. Pharm. Res. 1998, 21, 370–373. [Google Scholar] [CrossRef]
- Taqvi, S.I.H.; Ghayur, M.N.; Gilani, A.H.; Saify, Z.S.; Aftab, M.T. Synthesis and smooth muscle-selective relaxant activity of a piperidine analogue: 1-(4′-fluorophenacyl)-4-hydroxy-4-phenyl-piperidinium chloride. Arch. Pharm. Res. 2006, 29, 34–39. [Google Scholar] [CrossRef]
- Quasthoff, S.; Möckel, C.; Zieglgänsberger, W.; Schreibmayer, W. Tolperisone: A typical representative of a class of centrally acting muscle relaxants with less sedative side effects. CNS Neurosci. Ther. 2008, 14, 107–119. [Google Scholar] [CrossRef]
- DayioĞlu, H.; Yilmaz, A.; ErdoĞan, A.; Fatih, A.; Darcan, S. The investigation of the action mechanism of ammonium pyrrolodine dithiocarbamate on rat bladder smooth muscle contraction-relxation response. JSR-A 2020, 45, 28–49. [Google Scholar]
- DayioĞlu, H.; Yilmaz, A.; Keleş, Z.; Fatih, A.; Darcan, S. The investigation of the action mechanism of ammonium pyrrolidine dithiocarbamate on rat aorta smooth muscle contraction-relaxation responses. JSR-A 2020, 45, 50–69. [Google Scholar]
- Waddell, L.J.N.; Watts, O.F.B.; Saetang, P.; Rukachaisirikul, V.; Bates, R.W. Asperidine B: Total synthesis and structure correction. Tetrahedron Lett. 2020, 61, 152078. [Google Scholar] [CrossRef]
- Kirschstein, T.; Rehberg, M.; Bajorat, R.; Tokay, T.; Porath, K.; Köhling, R. High K+-induced contraction requires depolarization-induced Ca2+ release from internal stores in rat gut smooth muscle. Acta Pharmacol. Sin. 2009, 30, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Murthy, K.S. Signaling for contraction and relaxation in smooth muscle of the gut. Annu. Rev. Physiol. 2006, 68, 345–374. [Google Scholar] [CrossRef]
- Tanahashi, Y.; Komori, S.; Matsuyama, H.; Kitazawa, T.; Unno, T. Functions of muscarinic receptor subtypes in gastrointestinal smooth muscle: A review of studies with receptor-knockout mice. Int. J. Mol. Sci. 2021, 22, 926. [Google Scholar] [CrossRef]
- Unno, T.; Kwon, S.C.; Okamoto, H.; Irie, Y.; Kato, Y.; Matsuyama, H.; Komori, S. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br. J. Pharmacol. 2003, 139, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Dei, S.; Angeli, P.; Bellucci, C.; Buccioni, M.; Gualtieri, F.; Marucci, G.; Manetti, D.; Matucci, R.; Romanelli, M.N.; Scapecchi, S.; et al. Muscarinic subtype affinity and functional activity profile of 1-methyl-2-(2-methyl-1, 3-dioxolan-4-yl) pyrrolidine and 1-methyl-2-(2-methyl-1, 3-oxathiolan-5-yl) pyrrolidine derivatives. Biochem. Pharmacol. 2005, 69, 1637–1645. [Google Scholar] [CrossRef]
- Seno, K.; Okuno, T.; Nishi, K.; Murakami, Y.; Watanabe, F.; Matsuura, T.; Wada, M.; Fujii, Y.; Yamada, M.; Ogawa, T. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J. Med. Chem. 2000, 43, 1041–1044. [Google Scholar] [CrossRef]
- Murthy, K.S.; Makhlouf, G.M. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP-and cGMP-dependent protein kinases: Inhibitory phosphorylation of PLA2 by cyclic nucleotide-dependent protein kinases. J. Biol. Chem. 1998, 273, 34519–34526. [Google Scholar] [CrossRef] [Green Version]
- An, J.Y.; Yun, H.S.; Lee, Y.P.; Yang, S.J.; Shim, J.O.; Jeong, J.H.; Shin, C.Y.; Kim, J.H.; Kim, D.S.; Sohn, U.D. The intracellular pathway of the acetylcholine-induced contraction in cat detrusor muscle cells. Br. J. Pharmacol. 2002, 137, 1001–1010. [Google Scholar] [CrossRef] [Green Version]
- Buharalioğlu, C.K.; Akar, F. The reactivity of serotonin, acetylcholine and kcl-induced contractions to relaxant agents in the rat gastric fundus. Pharmacol. Res. 2002, 45, 325–331. [Google Scholar] [CrossRef]
- Nielsen-Kudsk, J.E. Potassium channel modulation: A new drug principle for regulation of smooth muscle contractility. Studies on isolated airways and arteries. Dan. Med. Bull. 1996, 43, 429–447. [Google Scholar]
- Vogalis, F. Potassium channels in gastrointestinal smooth muscle. J. Auton. Pharmacol. 2000, 20, 207–219. [Google Scholar] [CrossRef]
- Hermann, A.; Gorman, A.L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J. Gen. Physiol. 1981, 78, 87–110. [Google Scholar] [CrossRef] [Green Version]
- Andalib, P.; Consiglio, J.F.; Trapani, J.G.; Korn, S.J. The external TEA binding site and C-type inactivation in voltage-gated potassium channels. Biophys. J. 2004, 87, 3148–3161. [Google Scholar] [CrossRef] [Green Version]
- Al-Shboul, O. Contraction and relaxation signaling in gastrointestinal smooth muscle. ECGDS 2018, 5, 315–321. [Google Scholar]
- Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Bowornwiriyapan, K.; Muanprasat, C.; Srimaroeng, C.; Duangjai, A.; Sakayaroj, J. Lovastatin analogues from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. J. Nat. Prod. 2016, 79, 1500–1507. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Sakayaroj, J.; Srimaroeng, C.; Ontawong, A.; Duangjai, A.; Muangnil, P.; Muanprasat, C. Asperidines A–C, pyrrolidine and piperidine derivatives from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Bioorg. Med. Chem. 2018, 26, 4502–4508. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangjai, A.; Rukachaisirikul, V.; Sukpondma, Y.; Srimaroeng, C.; Muanprasat, C. Antispasmodic Effect of Asperidine B, a Pyrrolidine Derivative, through Inhibition of L-Type Ca2+ Channel in Rat Ileal Smooth Muscle. Molecules 2021, 26, 5492. https://doi.org/10.3390/molecules26185492
Duangjai A, Rukachaisirikul V, Sukpondma Y, Srimaroeng C, Muanprasat C. Antispasmodic Effect of Asperidine B, a Pyrrolidine Derivative, through Inhibition of L-Type Ca2+ Channel in Rat Ileal Smooth Muscle. Molecules. 2021; 26(18):5492. https://doi.org/10.3390/molecules26185492
Chicago/Turabian StyleDuangjai, Acharaporn, Vatcharin Rukachaisirikul, Yaowapa Sukpondma, Chutima Srimaroeng, and Chatchai Muanprasat. 2021. "Antispasmodic Effect of Asperidine B, a Pyrrolidine Derivative, through Inhibition of L-Type Ca2+ Channel in Rat Ileal Smooth Muscle" Molecules 26, no. 18: 5492. https://doi.org/10.3390/molecules26185492