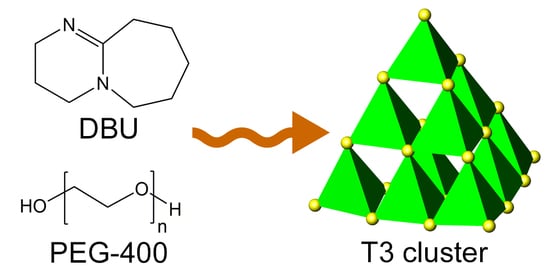
A Discrete Ligand-Free T3 Supertetrahedral Cluster of Gallium Sulfide
Abstract
:1. Introduction
2. Results
2.1. Crystal Structure of [C9H17N2]6[Ga10S16(SH)4]
2.2. Characterisation
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, P.; Bu, X.; Zheng, N. The interface chemistry between chalcogenide clusters and open framework chalcogenides. Acc. Chem. Res. 2005, 38, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bu, X.; Feng, P.; Wu, T. Metal chalcogenide supertetrahedral clusters: Synthetic control over assembly, dispersibility, and their functional applications. Acc. Chem. Res. 2020, 53, 2261−2272. [Google Scholar] [CrossRef]
- Levchenko, T.I.; Huang, Y.; Corrigan, J.F. Large metal chalcogenide clusters and their ordered superstructures via solvothermal and ionothermal syntheses. In Clusters—Contemporary Insight in Structure and Bonding; Dehnen, S., Ed.; Springer Structure and Bonding: Cham, Switzerland, 2016; Volume 174, pp. 269–319. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, Q.; Liu, Y.; Li, J.; Huang, X. Discrete supertetrahedral Tn chalcogenido clusters synthesized in ionic liquids: Crystal structures and photocatalytic activity. ChemPlusChem 2020, 85, 2487–2498. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, P.; Bu, X.; Wu, T. Atomically precise metal chalcogenide supertetrahedral clusters: Frameworks to molecules, and structure to function. Natl. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Bu, X.; Zheng, N.; Feng, P. Tetrahedral chalcogenide clusters and open frameworks. Chem. Eur. J. 2004, 10, 3356–3362. [Google Scholar] [CrossRef]
- Vaqueiro, P. Hybrid materials through linkage of chalcogenide tetrahedral clusters. Dalton Trans. 2010, 39, 5965–5972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, B.; Voelker, D.; Stiller, K.-O. Novel adamantane-like thio- and selenoanions from aqueous solution: Ga4S108−, In4S108−, In4Se108−. Inorg. Chim. Acta 1982, 65, L101–L102. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, S.; Wang, K.-Y.; Wang, J.; Cheng, L.; Zhu, J.-Y.; Zhao, Y.-M.; Wang, C. Mixed solvothermal synthesis of Tn cluster-based indium and gallium sulfides using versatile ammonia or amine structure-directing agents. Inorg. Chem. 2021, 60, 7115−7127. [Google Scholar] [CrossRef] [PubMed]
- Vaqueiro, P.; Romero, M.L. [Ga10S16(NC7H9)4]2−: A hybrid supertetrahedral nanocluster. Chem. Commun. 2007, 3282–3284. [Google Scholar] [CrossRef]
- Xiong, W.-W.; Li, J.-R.; Hu, B.; Tan, B.; Lia, R.-F.; Huang, X.-Y. Largest discrete supertetrahedral clusters synthesized in ionic liquids. Chem. Sci. 2012, 3, 1200–1204. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Bu, X.; Chau, V.; Feng, P. Largest molecular clusters in the supertetrahedral Tn series. J. Am. Chem. Soc. 2010, 132, 10823–10831. [Google Scholar] [CrossRef] [PubMed]
- Vaqueiro, P.; Romero, M.L. Three-dimensional gallium sulphide open frameworks. J. Phys. Chem. Solids 2007, 68, 1239–1243. [Google Scholar] [CrossRef]
- Zheng, N.; Bu, X.; Feng, P. Nonaqueous synthesis and selective crystallization of gallium sulfide clusters into three-dimensional photoluminescent superlattices. J. Am. Chem. Soc. 2003, 125, 1138–1139. [Google Scholar] [CrossRef] [PubMed]
- Vaqueiro, P.; Makin, S.; Tong, Y.; Ewing, S.J. A new class of hybrid super-supertetrahedral cluster and its assembly into a five-fold interpenetrating network. Dalton Trans. 2017, 46, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Vaqueiro, P.; Romero, M.L.; Rowan, B.C.; Richards, B.S. Arrays of chiral nanotubes and a layered coordination polymer containing gallium-sulphide supertetrahedral clusters. Chem. Eur. J. 2010, 16, 4462–4465. [Google Scholar] [CrossRef] [PubMed]
- Vaqueiro, P.; Romero, M.L. Gallium-sulfide supertetrahedral clusters as building blocks of covalent organic-inorganic networks. J. Am. Chem. Soc. 2008, 130, 9630–9631. [Google Scholar] [CrossRef] [Green Version]
- Peters, B.; Stuhrmann, G.; Mack, F.; Weigend, F.; Dehnen, S. Highly soluble supertetrahedra upon selective partial butylation of chalcogenido metalate clusters in ionic liquids. Angew. Chem. Int. Ed. 2021, in press. [Google Scholar] [CrossRef]
- Khokarale, S.G.; Mikkola, J.-P. Hydrogen sulfide gas capture by organic superbase 1,8-diazabicyclo-[5.4.0]-undec-7-ene through salt formation: Salt synthesis, characterization and application for CO2 capture. RSC Adv. 2018, 8, 18531–18541. [Google Scholar] [CrossRef] [Green Version]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef]
- Santner, S.; Heine, J.; Dehnen, S. Synthesis of crystalline chalcogenides in ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 876–893. [Google Scholar] [CrossRef]
- Xiong, W.-W.; Zhang, Q. Surfactants as promising media for the preparation of crystalline inorganic materials. Angew. Chem. Int. Ed. 2015, 54, 11616–11623. [Google Scholar] [CrossRef]
- Xiong, W.-W.; Miao, J.; Ye, K.; Wang, Y.; Liu, B.; Zhang, Q. Threading chalcogenide layers with polymer chains. Angew. Chem. Int. Ed. 2015, 54, 546–550. [Google Scholar] [CrossRef]
- Trikalitis, P.N.; Bakas, T.; Papaefthymiou, V.; Kanatzidis, M.G. Supramolecular assembly of hexagonal mesostructured germanium sulfide and selenide nanocomposites incorporating the biologically relevant Fe4S4 cluster. Angew. Chem. Int. Ed. 2000, 39, 4558–4562. [Google Scholar] [CrossRef]
- Ye, R.; Liu, B.-W.; Jiang, X.-M.; Lu, J.; Zeng, H.-Y.; Guo, G.-C. AMnAs3S6 (A = Cs, Rb): Phase-matchable infrared nonlinear optical functional motif [As3S6]3– obtained via surfactant–thermal method. ACS Appl. Mater. Interfaces 2020, 12, 53950–53956. [Google Scholar] [CrossRef]
- Yang, D.-D.; Song, Y.; Zhang, B.; Shen, N.-N.; Xu, G.-L.; Xiong, W.-W.; Huang, X.-Y. Exploring the surfactant–thermal synthesis of crystalline functional thioarsenates. Cryst. Growth Des. 2018, 18, 3255–3262. [Google Scholar] [CrossRef]
- Nie, L.; Liu, G.; Xie, J.; Lim, T.-L.; Armatas, G.S.; Xu, R.; Zhang, Q. Syntheses, crystal structures, and photocatalytic properties of two ammonium-directed Ag–Sb–S complexes. Inorg. Chem. Front. 2017, 4, 954–959. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, C.; Hou, P.; Zhi, M.; Zhou, C.; Chai, W.; Cheng, J.-W.; Liu, Y.; Zhang, Q. Preparation of porous three-dimensional quaternary thioantimonates(III) ACuSb2S4 (A = Rb, Cs) through a surfactant-thermal method. Chem. Asian J. 2015, 10, 2604–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, P.; Ding, J.; Liu, Y.; Xiong, W.-W.; Nie, L.; Wu, T.; Zhao, Y.; Tok, A.I.Y.; Zhang, Q. Surfactant-thermal syntheses, structures, and magnetic properties of Mn−Ge−sulfides/selenides. Inorg. Chem. 2014, 53, 10248–10256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Zhang, X.; Mu, W.-Q.; Luo, W.; Bian, G.-Q.; Zhu, Q.-Y.; Dai, J. Indium sulfide clusters integrated with 2,2’-bipyridine complexes. Dalton Trans. 2011, 40, 9746–9751. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.-X.; Zhu, Q.-Y.; Zhang, X.; Luo, W.; Mu, W.-Q.; Dai, J. Indium-sulfur supertetrahedral clusters integrated with a metal complex of 1,10-phenanthroline. Inorg. Chem. 2010, 49, 4385–4387. [Google Scholar] [CrossRef]
- Coles, S.J.; Gale, P.A. Changing and challenging times for service crystallography. Chem. Sci. 2012, 3, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786−790. [Google Scholar] [CrossRef] [Green Version]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS Version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]


| Crystallographic Formula | C54H102Ga10N12S20 |
| Mr | 2257.88 |
| Crystal habit | Colourless prism |
| Crystal system | Monoclinic |
| Space group | P21 |
| T/K | 100 |
| a/Å | 14.1675(3) |
| b/Å | 14.1898(3) |
| c/Å | 21.3007(4) |
| α/° | 90 |
| β/° | 90.5730(18) |
| γ/° | 90 |
| V/Å3 | 4281.95(15) |
| Z | 2 |
| ρcal/g cm−1 | 1.751 |
| Number of parameters | 536 |
| Rmerge | 0.0659 |
| R(I > 3.0σ(I)) | 0.0794 |
| Rw | 0.0595 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makin, S.; Vaqueiro, P. A Discrete Ligand-Free T3 Supertetrahedral Cluster of Gallium Sulfide. Molecules 2021, 26, 5415. https://doi.org/10.3390/molecules26175415
Makin S, Vaqueiro P. A Discrete Ligand-Free T3 Supertetrahedral Cluster of Gallium Sulfide. Molecules. 2021; 26(17):5415. https://doi.org/10.3390/molecules26175415
Chicago/Turabian StyleMakin, Sarah, and Paz Vaqueiro. 2021. "A Discrete Ligand-Free T3 Supertetrahedral Cluster of Gallium Sulfide" Molecules 26, no. 17: 5415. https://doi.org/10.3390/molecules26175415