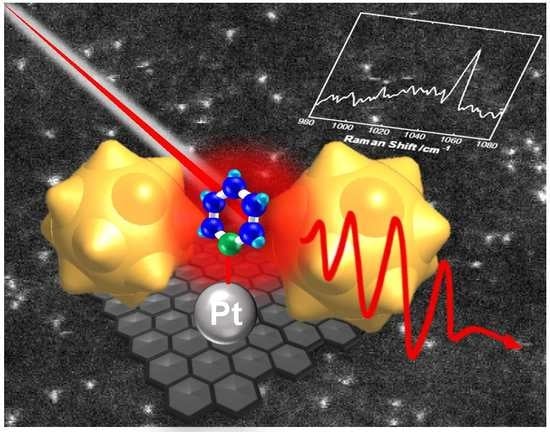
Development of Highly Sensitive Raman Spectroscopy for Subnano and Single-Atom Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication and Characterisation of Anisotropic Au SHINs
2.2. Enhanced Raman Sensitivity with Shell-Isolated Au Nanostars
2.3. SHINERS Study of Pyridine Molecules Adsorbed on Pt Subnano-Islands
2.4. Detection of the “Fingerprint” of a Single Atom
3. Materials and Methods
3.1. Materials
3.2. Characterisation Methodologies
3.3. Synthesis of Au Nanostars
3.4. Synthesis of Shell-Isolated Au Nanostars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Yamamoto, K.; Imaoka, T. Precision synthesis of subnanoparticles using dendrimers as a superatom synthesizer. Acc. Chem. Res. 2014, 47, 1127–1136. [Google Scholar] [CrossRef]
- Takahashi, M.; Imaoka, T.; Yamamoto, K. Reactivities of platinum subnanocluster catalysts for the oxidation reaction of alcohols. RSC Adv. 2015, 5, 100693. [Google Scholar] [CrossRef]
- Imaoka, T.; Kitazawa, H.; Chun, W.J.; Yamamoto, K. Finding the most catalytically active platinum clusters with low atomicity. Angew. Chem. Int. Ed. 2015, 54, 9810–9815. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Etchegoin, P.G.; LeRu, E.C. Basic electromagnetic theory of SERS. In Surface Enhanced Raman Spectroscopy; Schluecker, S., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 1–37. [Google Scholar]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley: Chichester, UK, 2006; pp. 73–132. [Google Scholar]
- Ozaki, Y.; Kneipp, K.; Aroca, R. Frontiers of Surface-Enhanced Raman Scattering, Single Nanoparticles and Single Cells; Wiley: Chichester, UK, 2014; pp. 19–57. [Google Scholar]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface-Enhanced Raman Scattering, Physics and Applications; Springer: Berlin/Heidelberg, Germany, 2006; pp. 19–65. [Google Scholar]
- Schluecker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Ren, B.; Wu, D.Y. Surface-enhanced Raman Scattering: From Noble to Transition Metals and from Rough Surfaces to Ordered Nanostructures. J. Phys. Chem. B 2002, 106, 9463–9483. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.L.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectro-chemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, C.; Sun, H.L.; Fu, G.; Chen, S.; Zhang, Y.J.; Chen, B.H.; Snema, H.R.; Yang, Z.L.; Li, J.F.; et al. In situ dynamic tracking of heterogeneous nanocatalytic processes by shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 15447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhang, X.G.; Wei, J.; Wang, C.; Chen, S.; Sun, H.L.; Wang, Y.H.; Chen, B.H.; Yang, Z.L.; Wu, D.Y.; et al. Revealing the role of interfacial properties on catalytic behaviors by in situ surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2018, 139, 10339–10346. [Google Scholar] [CrossRef] [PubMed]
- Kuzume, A.; Ozawa, M.; Tang, Y.; Yamada, Y.; Haruta, N.; Yama-moto, K. Ultrahigh sensitive Raman spectroscopy for subnanoscience: Direct observation of tin oxide clusters. Sci. Adv. 2019, 5, eaax6455. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.D.; Liu, B.J.; Li, J.F.; Yang, Z.L.; Ren, B.; Tian, Z.Q. SHINERS and plasmonic properties of Au core SiO2 shell nanopar-ticles with optimal core size and shell thickness. J. Raman Spectrosc. 2013, 44, 994–998. [Google Scholar] [CrossRef]
- Fang, P.P.; Li, J.F.; Yang, Z.L.; Li, L.M.; Ren, B.; Tian, Z.Q. Optimization of SERS activities of gold nanoparticles and gold-core-palladium-shell nanoparticles by controlling size and shell thickness. J. Raman Spectrosc. 2008, 39, 1679–1687. [Google Scholar] [CrossRef]
- Barbosa, S.; Agrawal, A.; Rodriguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-Marzán, L.M. Tuning size and sensing properties in colloidal gold nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef]
- Khoury, C.G.; Vo-Dinh, T. Gold nanostars for surface-enhanced Raman scattering: Synthesis, characterization and optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; Garcia de Abajo, F.J.; Liz-Marzán, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Langer, J.; Jimenez de Aberasturi, D.; Liz-Marzán, L.M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef] [Green Version]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation of PVP-protected metal nanoparticles in DMF. Langmuir 2002, 18, 2888–2894. [Google Scholar] [CrossRef]
- Wu, D.Y.; Duan, S.; Ren, B.; Tian, Z.Q. Density functional theory study of surface-enhanced Raman scattering spectra of pyridine adsorbed on noble transition metal surfaces. J. Raman Spectrosc. 2005, 36, 533–540. [Google Scholar] [CrossRef]
- Wu, D.Y.; Ren, B.; Jiang, Y.X.; Xu, X.; Tian, Z.Q. Density functional study and normal-mode analysis of the bindings and vibrational frequency shifts of the pyridine-M (M = Cu, Ag, Au, Cu+, Ag+, Au+ and Pt) complexes. J. Phys. Chem. A 2002, 106, 9042–9052. [Google Scholar] [CrossRef]
- Wu, D.Y.; Li, J.F.; Ren, B.; Tian, Z.Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 2008, 37, 1025–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.H.; Jin, X.; Meng, L.; Wang, X.; Su, H.S.; Yang, Z.L.; Williams, C.T.; Ren, B. Probing the electronic and catalytic properties of a bimetallic surface wih3 nm resolution. Nat. Nanotechnol. 2017, 12, 132–136. [Google Scholar] [CrossRef]
- Haq, S.; King, D.A. Configurational transitions of benzene and pyridine adsorbed on Pt{111} and Cu{110} surfaces: An infrared study. J. Phys. Chem. 1996, 100, 16957–16965. [Google Scholar] [CrossRef]
- Wei, J.; Qin, S.N.; Yang, J.; Ya, H.L.; Huang, W.H.; Zhang, H.; Hwang, B.J.; Tian, Z.Q.; Li, J.F. Probing single-atom catalysts and catalytic reaction processes by shell-isolated nanoparticle-enhanced Raman spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 9306–9310. [Google Scholar] [CrossRef]
- Hartman, T.; Weckhuysen, B.M. Thermally stable TiO2- and SiO2-shell-isolated Au nanoparticles for in situ plasmon-enhanced Raman spectroscopy of hydrogenation catalysts. Chem. Eur. J. 2018, 24, 37333741. [Google Scholar]
- Dutta, A.; Kuzume, A.; Rahaman, M.; Vesztergom, S.; Broekmann, P. Monitoring the chemical state of catalysts for CO2 electroreduc-tion: An in-operando study. ACS Catal. 2015, 5, 7498–7502. [Google Scholar] [CrossRef]
- Dutta, A.; Kuzume, A.; Kaliginedi, V.; Rahaman, M.; Sinev, I.; Ah-madi, M.; Cuenya, B.R.; Vesztergom, S.; Broekmann, P. Probing the chemical state of tin oxide NP catalysts during CO2 electroreduction: A complementary operando approach. Nano Energy 2018, 53, 828–840. [Google Scholar] [CrossRef] [Green Version]
- Rudnev, A.V.; Kuzume, A.; Fu, Y.; Wandlowski, T. CO oxidation on Pt(100): New insights based on combined voltammetric, microscopic and spectroscopic experiments. Electrochim. Acta 2014, 133, 132–145. [Google Scholar] [CrossRef]
- Li, J.F.; Tian, Z.D.; Li, S.B.; Anema, J.R.; Yang, Z.L.; Ding, Y.; Wu, Y.F.; Zeng, Y.M.; Chen, Q.Z.; Ren, B.; et al. Surface analysis using shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat. Protoc. 2013, 8, 52–65. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Haruta, N.; Kuzume, A.; Yamamoto, K. Development of Highly Sensitive Raman Spectroscopy for Subnano and Single-Atom Detection. Molecules 2021, 26, 5099. https://doi.org/10.3390/molecules26165099
Tang Y, Haruta N, Kuzume A, Yamamoto K. Development of Highly Sensitive Raman Spectroscopy for Subnano and Single-Atom Detection. Molecules. 2021; 26(16):5099. https://doi.org/10.3390/molecules26165099
Chicago/Turabian StyleTang, Yuansen, Naoki Haruta, Akiyoshi Kuzume, and Kimihisa Yamamoto. 2021. "Development of Highly Sensitive Raman Spectroscopy for Subnano and Single-Atom Detection" Molecules 26, no. 16: 5099. https://doi.org/10.3390/molecules26165099