Analytical Problems in Separation of Selenomethionine and Its Oxidative Product in HILIC HPLC
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Se in Tea Leaves and Infusions
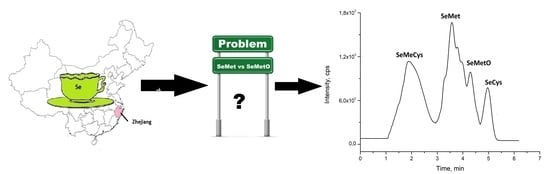
2.2. Speciation Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, R.; Liu, Y.; Zhou, Z. Selenium and Selenoproteins, from Structure, Function to Food Resource and Nutrition. Food Sci. Technol. Res. 2017, 23, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Kuršvietiené, L.; Mongirdiené, A.; Bernatoniené, J.; Šulinskiene, J.; Stanevičiené, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Pyrzynska, K. Edible plants enriched with selenium. J. Agric. Sci. Technol. 2014, 4, 627–632. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Puligundla, P.; Ko, S.; Wan, X.C. A review on selenium-enriched green tea: Fortification methods, Biological activties and application prospect. Sains Malays. 2014, 43, 1685–1692. [Google Scholar]
- Constantinescu-Aruxandei, D.; Frîncu, R.M.; Capră, L.; Oancea, F. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients 2018, 10, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Zhang, M.; Adhikari, B. Advances in selenium-enriched foods: From the farm to the fork. Trends Food Sci. Technol. 2018, 76, 1–5. [Google Scholar] [CrossRef]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Moreda-Piňeiro, J.; Moreda-Piňeiro, A.; Bermejo-Barrera, P. In vivo and in vitro testing for selenium and selenium com-pounds bioavailability assessment in foodstuff. Crit. Rev. Food Sci. Nutr. 2017, 57, 805–833. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutr. 2020, 61, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzyńska, K. Investigation of antioxidant activity of selenium compounds and their mixtures with tea polyphenols. Mol. Biol. Rep. 2019, 46, 3019–3024. [Google Scholar] [CrossRef] [Green Version]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Kápolna, E.; Fodor, P. Bioavailability of selenium from selenium-enriched green onions (Allium fistulosum) and chives (Allium schoenoprasum) after “in vitro” gastrointestinal digestion. Int. J. Food Sci. Nutr. 2007, 58, 282–298. [Google Scholar] [CrossRef]
- Liu, Q.; Bei, Y. Thermodynamics and dynamic kinetics of the oxidation of selenomethionine to methionine selenooxide: A DFT study. Prog. React. Kin. Mech. 2010, 36, 417–422. [Google Scholar] [CrossRef]
- Bierla, K.; Szpunar, J.; Yiannikouris, A.; Łobiński, R. Comprehensive speciation of selenium in selenium-rich yeast. TrAC Trends Anal. Chem. 2012, 41, 122–132. [Google Scholar] [CrossRef]
- Michalska-Kacymirow, M.; Kurek, E.; Smolis, A.; Wierzbicka, M.; Bulska, E. Biological and chemical investigation of Allium cepa L. response to selenium inorganic compounds. Anal. Bioanal. Chem. 2014, 406, 3717–3722. [Google Scholar] [CrossRef] [Green Version]
- Moreda-Piñeiro, J.; Sánchez-Piñero, J.; Mañana-López, A.; Turnes-Carou, I.; Alonso-Rodríguez, E.; López-Mahía, P.; Muniategui, S. Selenium species determination in foods harvested in Seleniferous soils by HPLC-ICP-MS after enzymatic hydrolysis assisted by pressurization and microwave energy. Food Res. Int. 2018, 111, 621–630. [Google Scholar] [CrossRef]
- Larsen, E.H.; Sloth, J.; Hansen, M.; Moesgaard, S. Selenium speciation and isotope composition in 77SeSe-enriched yeast using gradient elution HPLC and ICP-dynamic cell-MS. J. Anal. At. Spectrom. 2003, 18, 31–316. [Google Scholar] [CrossRef]
- Krata, A.A.; Wojciechowski, M.; Karasinski, J.; Bulska, E. Comparative study of high performance liquid chromatography species-specific and species-unspecific isotope dilution inductively coupled plasma mass spectrometry. A case study of selenomethionine and the origin of its oxidized form. Microchem. J. 2018, 143, 416–422. [Google Scholar] [CrossRef]
- Krause, R.J.; Glocke, S.C.; Sicuri, A.R.; Ripp, S.L.; Elfarra, A.A. Oxidative metabolism of seleno-L-methionine to L-methionine selenooxide by flavin-containing mono-oxygenases. Chem. Res. Toxicol. 2006, 19, 1643–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrero, Z.; Encinar, J.R.; Madrid, Y.; Cámara, C.; Zayas, Z.P. Application of species-specific isotope dilution analysis to the correction for selenomethionine oxidation in Se-enriched yeast sample extracts during storage. J. Anal. At. Spectrom. 2007, 22, 1061–1066. [Google Scholar] [CrossRef]
- LeBlanc, K.L.; Kumkrong, P.; Mercier, P.H.; Mester, Z. Selenium analysis in waters. Part 2: Speciation methods. Sci. Total Environ. 2018, 640–641, 1635–1651. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Liquid chromatographic analysis of selenium species in plant materials. TrAC Trends Anal. Chem. 2019, 111, 128–138. [Google Scholar] [CrossRef]
- Bierla, K.; Godin, S.; Łobiński, R.; Szpunar, J. Advances in electrospray mass spectrometry for the selenium speciation: Focus on Se-rich yeast. TrAC Trends Anal. Chem. 2018, 104, 87–94. [Google Scholar] [CrossRef]
- Pedrero, Z.; Encinar, J.R.; Madrid, Y.; Cámara, C. Identification of selenium species in selenium-enriched Lens esculenta plants by using two-dimensional liquid chromatography-inductively coupled plasma mass spectrometry and [77Se]selenomethionine selenium oxide spikes. J. Chromatogr. A 2007, 1139, 247–253. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Hydrophilic interaction liquid chromatography in the speciation analysis of selenium. J. Chromatogr. B 2018, 1074, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [Green Version]
- Dejaegher, B.; Mangelings, D.; Heyden, Y.V. Method development for HILIC assays. J. Sep. Sci. 2008, 31, 1438–1448. [Google Scholar] [CrossRef]
- Meriö-Talvio, H.; Dou, J.; Vuorinen, T.; Pitkänen, L. Fast HILIC Method for Separation and Quantification of Non-Volatile Aromatic Compounds and Monosaccharides from Willow (Salix sp.) Bark Extract. Appl. Sci. 2021, 11, 3808. [Google Scholar] [CrossRef]
- Bierła, K.; Suzuki, N.; Ogra, Y.; Szpunar, J.; Łobiński, R. Identification and determination of selenohomolanthionine–The major selenium compound in Torula yeast. Food Chem. 2017, 237, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Biesaga, M.; Pyrzyńska, K. Retention Study of Flavonoids under Different Chromatographic Modes. J. Chromatogr. Sci. 2015, 54, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Senanayake, S.P.J.N. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Yoshida, M.; Kimura, Y.; Abe, M.; Ando, T.; Tachi, H.; Fukunaga, K. Quantitative Evaluation of Selenium Contained in Tea by High Performance Liquid Chromatography. J. Nutr. Sci. Vitaminol. 2001, 47, 248–252. [Google Scholar] [CrossRef]
- Wen, S.; Zhu, X.; Wei, Y.; Wu, S. Cloud point extraction-inductively coupled plasma mass spectrometry for separa-tion/analysis of aqueous-exchangeable and unaqueous-exchengeable selenium in tea samples. Food Anal. Methods. 2013, 6, 506–511. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, S.; Lu, D. Solidified floating organic drop microextraction for speciation of selenium and its distribution in selenium-rich tea leaves and tea infusion by electrothermal vapourisation inductively coupled plasma mass spectrometry. Food Chem. 2015, 169, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Collings, L.; Harvey, J.; King, M.; Hooper, L.; Bouwman, J.; Gurinovic, M.; Fairweather-Tait, S.J. EURRECA—Estimating selenium requirements for deriving dietary reference values. Crit. Rev. Food Sci. Nutr. 2013, 53, 1077–1096. [Google Scholar] [CrossRef]
- Sentkowska, A. Content of selenoaminoacids and catechins in Chinese green teas. Eur. Food Res. Technol. 2021, 247, 613–622. [Google Scholar] [CrossRef]
- Yu, F.; Sheng, J.; Xu, J.; An, X.; Hu, Q. Antioxidant activities of crude tea polyphenols, polysaccharides and proteins of selenium-enriched tea and regular green tea. Eur. Food Res. Technol. 2006, 225, 843–848. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Y.; Catron, B.; Chan, Q.; Hu, Q.; Caruso, J.A. Identification of selenium compounds using HPLC-ICPMS and nano-ESI-MS in selenium-enriched rice via foliar application. J. Anal. At. Spectrom. 2009, 24, 1657–1664. [Google Scholar] [CrossRef]
- Obieziurska-Fabisiak, M.; Pacuła, A.J.; Capoccia, L.; Drogosz-Stachowicz, J.; Janecka, A.; Santi, C.; Ścianowski, J. Phenylselanyl group incorporation for “glutathione peroxidase-like” activity modulation. Molecules 2020, 25, 3354. [Google Scholar] [CrossRef] [PubMed]
- Chéry, C.C.; Dumont, D.; Moens, L.; Vanhaecke, F.; Cornelis, R. Influence of reducing agents on the integrity of selenocompounds. Exploratory work for selenoproteome analysis. J. Anal. At. Spectrom. 2005, 349, 620–625. [Google Scholar] [CrossRef]
- Krause, R.J.; Elffara, A.A. Reduction of L-methionine selenoxide to seleno-L-methionine by endogenous thiols, ascorbic acid, or methimazole. Biochem. Pharmacol. 2009, 77, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.G.; Kumar, P.; Iwaoka, M.; Priyadarsini, I. Free radical induced selenoxide formation in isomeric organoselenium compounds: The effect of chemical structures on antioxidant activity. NJC 2019, 43, 13357–13362. [Google Scholar] [CrossRef]
- Buszewski, B.; Bocian, S.; Rychlicki, G.; Vajda, P.; Felinger, A. Study of solvent adsorption on chemically bonded stationary phases by microcalorimetry and liquid chromatography. J. Colloid Interface Sci. 2010, 349, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Letzel, T. Main Interactions and Influences of the Chromatographic Parameters in HILIC Separations. J. Chromatogr. Sci. 2013, 51, 684–693. [Google Scholar] [CrossRef]






| Tea Samples | Dry Leaves (µg g−1) * | Infusion (µg L−1) | Extraction (%) |
|---|---|---|---|
| Lung-Ching −1 | 4.63 ± 0.05 | 13.8 ± 0.110 | 29.8 |
| Lung-Ching −2 | 5.13 ± 0.03 | 17.3 ± 0.123 | 33.7 |
| Lung-Ching −3 | 4.73 ± 0.04 | 14.2 ± 0.100 | 30.0 |
| Lung-Ching −4 | 4.60 ± 0.05 | 12.5 ± 0.100 | 27.2 |
| Yunnan −1 | 3.21 ± 0.02 | 6.83 ± 0.105 | 21.3 |
| Yunnan −2 | 3.36 ± 0.08 | 7.31 ± 0.011 | 21.8 |
| Dilmah | 3.09 ± 0.09 | 5.63 ± 0.003 | 18.2 |
| Lipton | 2.96 ± 0.07 | 4.37 ± 0.020 | 14.8 |
| Analyte Abbreviation | Slope | R2 | LOD [µg/L] | LOQ [µg/L] | RSD % |
|---|---|---|---|---|---|
| SeMet | 1,636,351 | 0.9999 | 0.05 | 0.10 | 2.2 |
| SeMetO | 1,473,217 | 0.9999 | 0.05 | 0.10 | 2.0 |
| MeSeCys | 1,223,582 | 0.9999 | 0.05 | 0.10 | 2.2 |
| SeCys | 18,224 | 0.9998 | 0.06 | 0.30 | 2.1 |
| Se(IV) | 11,304 | 0.9989 | 0.06 | 0.30 | 2.3 |
| Se(VI) | 59,310 | 0.9997 | 0.06 | 0.15 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentkowska, A.; Pyrzynska, K. Analytical Problems in Separation of Selenomethionine and Its Oxidative Product in HILIC HPLC. Molecules 2021, 26, 5073. https://doi.org/10.3390/molecules26165073
Sentkowska A, Pyrzynska K. Analytical Problems in Separation of Selenomethionine and Its Oxidative Product in HILIC HPLC. Molecules. 2021; 26(16):5073. https://doi.org/10.3390/molecules26165073
Chicago/Turabian StyleSentkowska, Aleksandra, and Krystyna Pyrzynska. 2021. "Analytical Problems in Separation of Selenomethionine and Its Oxidative Product in HILIC HPLC" Molecules 26, no. 16: 5073. https://doi.org/10.3390/molecules26165073