Controlling the Carbon-Bio Interface via Glycan Functional Adlayers for Applications in Microbial Fuel Cell Bioanodes
Abstract
:1. Introduction
2. Results
2.1. Surface Functionalization Using Phenyl-Glycosides
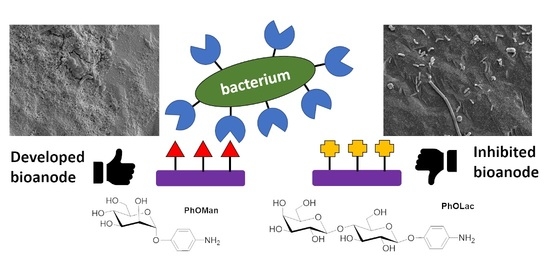
2.2. MFC Device Testing with Glycan-Modified Bioanodes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Surface Functionalization
4.3. Characterization
4.4. Microbial Fuel Cell Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Logan, B.E. Microbial Fuel Cells; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Iannaci, A.; Pepè Sciarria, T.; Mecheri, B.; Adani, F.; Licoccia, S.; D’Epifanio, A. Power generation using a low-cost sulfated zirconium oxide based cathode in single chamber microbial fuel cells. J. Alloys Compd. 2017, 693, 170–176. [Google Scholar] [CrossRef]
- Virdis, B.; Freguia, S.; Rozendal, R.A.; Rabaey, K.; Yuan, Z.; Keller, J. 4.18-Microbial Fuel Cells. In Treatise on Water Science; Wilderer, P., Ed.; Elsevier Science: Amsterdam, The Netherlands; London, UK, 2011; pp. 641–665. [Google Scholar]
- Popp, A.; Lotze-Campen, H.; Bodirsky, B. Food consumption, diet shifts and associated non-CO2 greenhouse gases from agricultural production. Glob. Environ. Chang. 2010, 20, 451–462. [Google Scholar] [CrossRef]
- Taboada-Santos, A.; Lema, J.M.; Carballa, M. Energetic and economic assessment of sludge thermal hydrolysis in novel wastewater treatment plant configurations. Waste Manag. 2019, 92, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ec.europa.eu/info/strategy/recovery-plan-europe_en (accessed on 24 June 2021).
- Bose, D.; Sridharan, S.; Dhawan, H.; Vijay, P.; Gopinath, M. Biomass derived activated carbon cathode performance for sustainable power generation from Microbial Fuel Cells. Fuel 2019, 236, 325–337. [Google Scholar] [CrossRef]
- Krieg, T.; Mayer, F.; Sell, D.; Holtmann, D. Insights into the applicability of microbial fuel cells in wastewater treatment plants for a sustainable generation of electricity. Environ. Technol. 2019, 40, 1101–1109. [Google Scholar] [CrossRef]
- Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Nieto-Monge, M.J.; Lazaro, M.J.; Licoccia, S. Iron-Based Electrocatalysts Supported on Nanostructured Carbon to Enhance Oxygen Reduction in Microbial Fuel Cells. ECS Trans. 2016, 72, 9–15. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Sun, M.; Zhai, L.F.; Li, W.W.; Yu, H.Q. Harvest and utilization of chemical energy in wastes by microbial fuel cells. Chem. Soc. Rev. 2016, 45, 2847–2870. [Google Scholar] [CrossRef]
- Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Mauri, A.; Licoccia, S. Carbon-Supported Zirconium Oxide as a Cathode for Microbial Fuel Cell Applications. ChemPlusChem 2016, 81, 80–85. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.; Rabaey, K.; Keller, J.; Buisman, C.J. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Iannaci, A.; Myles, A.; Flinois, T.; Behan, J.A.; Barrière, F.; Scanlan, E.M.; Colavita, P.E. Tailored glycosylated anode surfaces: Addressing the exoelectrogen bacterial community via functional layers for microbial fuel cell applications. Bioelectrochemistry 2020, 136, 107621. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Aghababaie, M.; Farhadian, M.; Jeihanipour, A.; Biria, D. Effective factors on the performance of microbial fuel cells in wastewater treatment—A review. Environ. Technol. Rev. 2015, 4, 71–89. [Google Scholar] [CrossRef]
- Prévoteau, A.; Rabaey, K. Electroactive Biofilms for Sensing: Reflections and Perspectives. ACS Sens. 2017, 2, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Domìnguez, C.; Behan, J.A.; Colavita, P.E. Electrocatalysis at nanocarbons: Model systems and applications in energy conversion. In Nanocarbon Electrochemistry; Yang, N., Zhao, G., Foord, J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2020; pp. 201–249. [Google Scholar]
- Liu, J.L.; Lowy, D.A.; Baumann, R.G.; Tender, L.M. Influence of anode pretreatment on its microbial colonization. J. Appl. Microbiol. 2007, 102, 177–183. [Google Scholar] [CrossRef]
- Kumar, G.G.; Sarathi, V.G.S.; Nahm, K.S. Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens. Bioelectron. 2013, 43, 461–475. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Scott, K.; Curtis, T.P.; Head, I.M. Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem. Eng. J. 2010, 156, 40–48. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Wang, X.; Wang, X.; Li, W. Porous carbon with defined pore size as anode of microbial fuel cell. Biosens. Bioelectron. 2015, 69, 135–141. [Google Scholar] [CrossRef]
- Zhu, N.; Chen, X.; Zhang, T.; Wu, P.; Li, P.; Wu, J. Improved performance of membrane free single-chamber air-cathode microbial fuel cells with nitric acid and ethylenediamine surface modified activated carbon fiber felt anodes. Bioresour. Technol. 2011, 102, 422–426. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Wang, H.; Jin, T. Anode modification by electrochemical oxidation: A new practical method to improve the performance of microbial fuel cells. Biochem. Eng. J. 2012, 60, 151–155. [Google Scholar] [CrossRef]
- Tang, X.; Guo, K.; Li, H.; Du, Z.; Tian, J. Electrochemical treatment of graphite to enhance electron transfer from bacteria to electrodes. Bioresour. Technol. 2011, 102, 3558–3560. [Google Scholar] [CrossRef]
- Cercado-Quezada, B.; Delia, M.L.; Bergel, A. Electrochemical micro-structuring of graphite felt electrodes for accelerated formation of electroactive biofilms on microbial anodes. Electrochem. Commun. 2011, 13, 440–443. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Commault, A.S.; Barrière, F.; Lapinsonnière, L.; Lear, G.; Bouvier, S.; Weld, R.J. Influence of inoculum and anode surface properties on the selection of Geobacter-dominated biofilms. Bioresour. Technol. 2015, 195, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Picot, M.; Lapinsonnière, L.; Rothballer, M.; Barrière, F. Graphite anode surface modification with controlled reduction of specific aryl diazonium salts for improved microbial fuel cells power output. Biosens. Bioelectron. 2011, 28, 181–188. [Google Scholar] [CrossRef]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of Surface Charge and Hydrophobicity on Anodic Biofilm Formation, Community Composition, and Current Generation in Bioelectrochemical Systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Lapinsonnière, L.; Picot, M.; Poriel, C.; Barrière, F. Phenylboronic Acid Modified Anodes Promote Faster Biofilm Adhesion and Increase Microbial Fuel Cell Performances. Electroanalysis 2013, 25, 601–605. [Google Scholar] [CrossRef]
- Smida, H.; Lebègue, E.; Bergamini, J.-F.; Barrière, F.; Lagrost, C. Reductive electrografting of in situ produced diazopyridinium cations: Tailoring the interface between carbon electrodes and electroactive bacterial films. Bioelectrochemistry 2018, 120, 157–165, Erratum in 2019, 125, 70, doi:10.1016/j.bioelechem.2018.07.012. [Google Scholar] [CrossRef]
- Şen-Doğan, B.; Okan, M.; Afşar-Erkal, N.; Özgür, E.; Zorlu, Ö.; Külah, H. Enhancement of the Start-Up Time for Microliter-Scale Microbial Fuel Cells (µMFCs) via the Surface Modification of Gold Electrodes. Micromachines 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Babanova, S.; Artyushkova, K.; Cornejo, J.A.; Ista, L.; Bretschger, O.; Marsili, E.; Atanassov, P.; Schuler, A.J. Influence of anode surface chemistry on microbial fuel cell operation. Bioelectrochemistry 2015, 106, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, D.F.; Elimelech, M. Role of type 1 fimbriae and mannose in the development of Escherichia coli K12 biofilm: From initial cell adhesion to biofilm formation. Biofouling 2009, 25, 401–411. [Google Scholar] [CrossRef]
- Klemm, P.; Schembri, M.A. Bacterial adhesins: Function and structure. Int. J. Med. Microbiol. 2000, 290, 27–35. [Google Scholar] [CrossRef]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial Adhesins in Host-Microbe Interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef] [Green Version]
- Purcell, S.C.; Godula, K. Synthetic glycoscapes: Addressing the structural and functional complexity of the glycocalyx. Interface Focus 2019, 9, 20180080. [Google Scholar] [CrossRef] [Green Version]
- Angione, M.D.; Duff, T.; Bell, A.P.; Stamatin, S.N.; Fay, C.; Diamond, D.; Scanlan, E.M.; Colavita, P.E. Enhanced Antifouling Properties of Carbohydrate Coated Poly(ether sulfone) Membranes. ACS Appl. Mater. Interfaces 2015, 7, 17238–17246. [Google Scholar] [CrossRef] [Green Version]
- Behan, J.A.; Myles, A.; Iannaci, A.; Whelan, E.; Scanlan, E.M.; Colavita, P.E. Bioinspired Electro-Permeable Glycans at Carbon: Fouling Control for Sensing in Complex Matrices. Carbon 2019, 158, 519–526. [Google Scholar] [CrossRef]
- Myles, A.; Haberlin, D.; Esteban-Tejeda, L.; Angione, M.D.; Browne, M.P.; Hoque, M.K.; Doyle, T.K.; Scanlan, E.M.; Colavita, P.E. Bioinspired aryldiazonium carbohydrate coatings: Reduced adhesion of foulants at polymer and stainless steel surfaces in a marine environment. ACS Sus. Chem. Eng. 2017, 6, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Zen, F.; Angione, M.D.; Behan, J.A.; Cullen, R.J.; Duff, T.; Vasconcelos, J.M.; Scanlan, E.M.; Colavita, P.E. Modulation of Protein Fouling and Interfacial Properties at Carbon Surfaces via Immobilization of Glycans Using Aryldiazonium Chemistry. Sci. Rep. 2016, 6, 24840. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Sheng, G.P.; Luo, H.W.; Li, W.W.; Wang, L.F.; Yu, H.Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef]
- Santoro, C.; Stadlhofer, A.; Hacker, V.; Squadrito, G.; Schröder, U.; Li, B. Activated carbon nanofibers (ACNF) as cathode for single chamber microbial fuel cells (SCMFCs). J. Power Sources 2013, 243, 499–507. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, F.; Zhang, X.; Liang, P.; Huang, X.; Logan, B.E. Use of Pyrolyzed Iron Ethylenediaminetetraacetic Acid Modified Activated Carbon as Air–Cathode Catalyst in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2013, 5, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Kimata, K.; Aiba, H. Mechanism responsible for glucose–lactose diauxie in Escherichia coli: Challenge to the cAMP model. Genes Cells 1996, 1, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monod, J. From Enzymatic Adaptation to Allosteric Transitions. Science 1966, 154, 475. [Google Scholar] [CrossRef] [PubMed]
- Lawford, H.G.; Rousseau, J.D. Relative rates of sugar utilization by an ethanologenic recombinantEscherichia coli using mixtures of glucose, mannose, and xylose. Appl. Biochem. Biotechnol. 1994, 45, 367–381. [Google Scholar] [CrossRef]
- Jayasundara, D.R.; Duff, T.; Angione, M.D.; Bourke, J.; Murphy, D.M.; Scanlan, E.M.; Colavita, P.E. Carbohydrate coatings via aryldiazonium chemistry for surface biomimicry. Chem. Mater. 2013, 25, 4122–4128. [Google Scholar] [CrossRef]
- Myles, A.; Behan, J.A.; Twamley, B.; Colavita, P.E.; Scanlan, E.M. Spontaneous aryldiazonium grafting for the preparation of functional cyclodextrin-modified materials. ACS Appl. Bio Mater. 2018, 1, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Brooksby, P.A.; Downard, A.J. Electrochemical and Atomic Force Microscopy study of carbon surface modification via diazonium reduction in aqueous and acetonitrile solutions. Langmuir 2004, 20, 5038–5045. [Google Scholar] [CrossRef]
- Anariba, F.; DuVall, S.H.; McCreery, R.L. Mono- and Multilayer formation by diazonium reduction on carbon surfaces monitored with atomic force microscopy “scratching”. Anal. Chem. 2003, 75, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Hsu, L.H.-H.; Kavanagh, P.; Barrière, F.; Lens, P.N.; Lapinsonnière, L.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 0024. [Google Scholar] [CrossRef]
- Otto, K. Biophysical approaches to study the dynamic process of bacterial adhesion. Res. Microbiol. 2008, 159, 415–422. [Google Scholar] [CrossRef]
- Jayasundara, D.R.; Cullen, R.J.; Colavita, P.E. In situ and real time characterization of spontaneous grafting of aryldiazonium salts at carbon surfaces. Chem. Mater. 2013, 25, 1144–1152. [Google Scholar] [CrossRef]
- Matko, V.; Milanovič, M. Detection Principles of Temperature compensated oscillators with reactance influence on piezoelectric resonator. Sensors 2020, 20, 802. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Thompson, M. Surface morphology and the response of the thickness-shear mode acoustic wave sensor in liquids. Langmuir 1993, 9, 1990–1994. [Google Scholar] [CrossRef]
- Yang, M.; Thompson, M.; Duncan-Hewitt, W.C. Interfacial properties and the response of the thickness-shear-mode acoustic wave sensor in liquids. Langmuir 1993, 9, 802–811. [Google Scholar] [CrossRef]
- Jayasundara, D.R.; Cullen, R.J.; Soldi, L.; Colavita, P.E. In Situ Studies of the Adsorption Kinetics of 4-Nitrobenzenediazonium Salt on Gold. Langmuir 2011, 27, 13029–13036. [Google Scholar] [CrossRef]
- Xi, J.; Chen, J. Quartz Crystal Microbalance in Cell Biology Studies. J. Biochips Tiss. Chips 2013, s5, 1–9. [Google Scholar] [CrossRef]
- Babauta, J.T.; Beasley, C.A.; Beyenal, H. Investigation of electron transfer by geobacter sulfurreducens biofilms by using an electrochemical quartz crystal microbalance. ChemElectroChem 2014, 1, 2007–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, P.; Johannsmann, D. Environmental-stress-induced increased softness of electroactive biofilms, determined with a torsional quartz crystal microbalance. Anal. Chem. 2019, 91, 14476–14481. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, X.; Luo, Y.; Sun, D.; Call, D.F.; Logan, B.E. Improving startup performance with carbon mesh anodes in separator electrode assembly microbial fuel cells. Bioresour. Technol. 2013, 133, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An overview of electron acceptors in microbial fuel cells. Front. Microbiol. 2017, 8, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tommasi, T.; Salvador, G.P.; Quaglio, M. New insights in Microbial Fuel Cells: Novel solid phase anolyte. Sci. Rep. 2016, 6, 29091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friman, H.; Schechter, A.; Ioffe, Y.; Nitzan, Y.; Cahan, R. Current production in a microbial fuel cell using a pure culture of C upriavidus basilensis growing in acetate or phenol as a carbon source. Microb. Biotechnol. 2013, 6, 425–434. [Google Scholar] [CrossRef]
- Straight, J.V.; Ramkrishna, D.; Parulekar, S.J.; Jansen, N.B. Bacterial growth on lactose: An experimental investigation. Biotechnol. Bioeng. 1989, 34, 705–716. [Google Scholar] [CrossRef]
- Hofsten, B.V. The inhibitory effect of galactosides on the growth of Escherichia coli. Biochim. Biophys. Acta 1961, 48, 164–171. [Google Scholar] [CrossRef]
- Liang, M.N.; Smith, S.P.; Metallo, S.J.; Choi, I.S.; Prentiss, M.; Whitesides, G.M. Measuring the forces involved in polyvalent adhesion of uropathogenic Escherichia coli to mannose-presenting surfaces. Proc. Natl. Acad. Sci. USA 2000, 97, 13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narang, A.; Pilyugin, S.S. Bacterial gene regulation in diauxic and non-diauxic growth. J. Theor. Biol. 2007, 244, 326–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban-Tejeda, L.; Duff, T.; Ciapetti, G.; Daniela Angione, M.; Myles, A.; Vasconcelos, J.M.; Scanlan, E.M.; Colavita, P.E. Stable hydrophilic poly(dimethylsiloxane) via glycan surface functionalization. Polymer 2016, 106, 1–7. [Google Scholar] [CrossRef]








| Sample | Cathodic Charge Density (C m−2) |
|---|---|
| ITO_Lac | 3.8 ± 0.5 |
| ITO_Man | 3.0 ± 0.6 |
| GR_Lac | 32 ± 6 |
| GR_Man | 85 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannaci, A.; Myles, A.; Philippon, T.; Barrière, F.; Scanlan, E.M.; Colavita, P.E. Controlling the Carbon-Bio Interface via Glycan Functional Adlayers for Applications in Microbial Fuel Cell Bioanodes. Molecules 2021, 26, 4755. https://doi.org/10.3390/molecules26164755
Iannaci A, Myles A, Philippon T, Barrière F, Scanlan EM, Colavita PE. Controlling the Carbon-Bio Interface via Glycan Functional Adlayers for Applications in Microbial Fuel Cell Bioanodes. Molecules. 2021; 26(16):4755. https://doi.org/10.3390/molecules26164755
Chicago/Turabian StyleIannaci, Alessandro, Adam Myles, Timothé Philippon, Frédéric Barrière, Eoin M. Scanlan, and Paula E. Colavita. 2021. "Controlling the Carbon-Bio Interface via Glycan Functional Adlayers for Applications in Microbial Fuel Cell Bioanodes" Molecules 26, no. 16: 4755. https://doi.org/10.3390/molecules26164755