Characterization of a Water-Dispersed Biodegradable Polyurethane-Silk Composite Sponge Using 13C Solid-State Nuclear Magnetic Resonance as Coating Material for Silk Vascular Grafts with Small Diameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biodegradable PU and Water-Dispersed Biodegradable PU Samples
2.3. Characterization of Water-Dispersed Biodegradable PU Sample
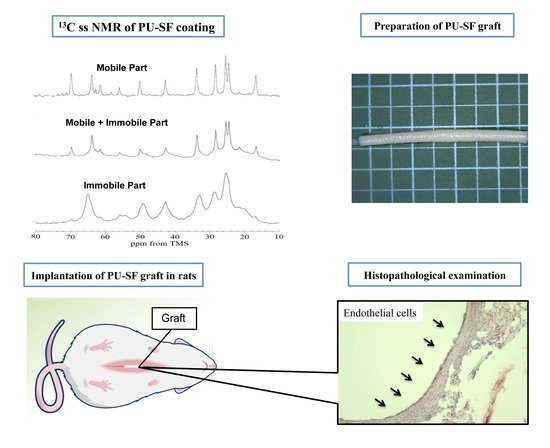
2.4. Preparation of a Biodegradable PU-SF Film (Sample I), Biodegradable PU-SF Precipitate (Sample II), and Water-Dispersed Biodegradable PU-SF Sponge (Sample III)
2.5. 13C Solid-State NMR Observations of the PU-SF Composite Materials in Dry and Hydrated States
2.6. In Vivo Degradation Test of a Water-Dispersed Biodegradable PU-SF Sponge (Sample III)
2.7. Preparation of SF Vascular Grafts Coated with a Water-Dispersed SF-PU Composite Sponge (PU-SF-Coated SF Grafts) and Water-Dispersed PU Sponge Only (PU-Coated SF Grafts)
2.8. Mechanical Properties of the SF Double-Raschel Knitted Tube Coated with a Water-Dispersed PU-SF Composite Sponge and SF Double-Raschel Knitted Tube without Coating
2.9. Implantation of the SF Vascular Grafts in Rats
2.10. Histopathological Examination
2.11. Animals
3. Results and Discussion
3.1. Examination of Biodegradable PU-SF Composite Materials for Coating Knitted SF Grafts
3.1.1. 13C CP/MAS NMR Spectra of Samples I and II in Dry States
3.1.2. 13C Solid-State NMR Spectra of Sample II in Hydrated States
3.1.3. 13C Solid-State NMR Spectra of Sample III in Hydrated States
3.2. Implantation Experiments with Rats
3.2.1. Water-Dispersed Biodegradable PU-SF Composite Sponge
3.2.2. Tensile Testing of the SF Double-Raschel Knitted Graft without and with PU-SF Composite Sponge
3.2.3. SF Grafts Coated with Only the PU and Water-Dispersed Biodegradable PU-SF Composite Sponges
3.3. Histopathological Examination
3.3.1. Water-Dispersed Biodegradable PU-SF Composite Sponge
3.3.2. SF Grafts Coated with Water-Dispersed Biodegradable PU and PU-SF Composite Sponges Only
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, G.A.; Chalmers, N.; Georgiadis, G.S.; Lazarides, M.K.; Antoniou, S.A.; Serracino-Inglott, F.; Smyth, J.V.; Murray, D. A meta-analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J. Vasc. Surg. 2013, 57, 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, M.S. Critical appraisal of surgical revascularization for critical limb ischemia. J. Vasc. Surg. 2013, 57, 8S–13S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seal, B.; Otero, T.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Begovac, P.C.; Thomson, R.C.; Fisher, J.L.; Hughson, A.; Gällhagen, A. Improvements in GORE-TEX® vascular graft performance by Carmeda® bioactive surface heparin immobilization. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Heise, M.; Schmidmaier, G.; Husmann, I.; Heidenhain, C.; Schmidt, J.; Neuhaus, P.; Settmacher, U. PEG-hirudin/iloprost Coating of Small Diameter ePTFE Grafts Effectively Prevents Pseudointima and Intimal Hyperplasia Development. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Yokota, T.; Ichikawa, H.; Matsumiya, G.; Kuratani, T.; Sakaguchi, T.; Iwai, S.; Shirakawa, Y.; Torikai, K.; Saito, A.; Uchimura, E.; et al. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J. Thorac. Cardiovasc. Surg. 2008, 136, 900–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, S.; Boey, F.; Lao, L.L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008, 33, 853–874. [Google Scholar] [CrossRef]
- Tatterton, M.; Wilshaw, S.-P.; Ingham, E.; Homer-Vanniasinkam, S. The Use of Antithrombotic Therapies in Reducing Synthetic Small-Diameter Vascular Graft Thrombosis. Vasc. Endovascular Surg. 2012, 46, 212–222. [Google Scholar] [CrossRef]
- Asakura, T.; Kaplan, D.L. Silk production and processing. In Encyclopedia of Agricultural Science; Arutzen, C.J., Ed.; Academic Press: New York, NY, USA, 1994; Volume 4, pp. 1–11. [Google Scholar]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Lovett, M.; Cannizzaro, C.; Daheron, L.; Messmer, B.; Vunjak-Novakovic, G.; Kaplan, D.L. Silk fibroin microtubes for blood vessel engineering. Biomaterials 2007, 28, 5271–5279. [Google Scholar] [CrossRef] [Green Version]
- Lovett, M.; Eng, G.; Kluge, J.; Cannizzaro, C.; Vunjak-Novakovic, G.; Kaplan, D.L. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010, 6, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipe, E.C.; Santos, M.; Hung, J.; Lee, B.S.L.; Yang, N.; Chan, A.H.P.; Ng, M.K.C.; Rnjak-Kovacina, J.; Wise, S.G. Rapid Endothelialization of Off-the-Shelf Small Diameter Silk Vascular Grafts. JACC Basic Transl. Sci. 2018, 3, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, H.; Fan, Y. Silk fibroin for vascular regeneration. Microsc. Res. Tech. 2017, 80, 280–290. [Google Scholar] [CrossRef]
- Asakura, T.; Tanaka, T.; Tanaka, R. Advanced Silk Fibroin Biomaterials and Application to Small-Diameter Silk Vascular Grafts. ACS Biomater. Sci. Eng. 2019, 5, 5561–5577. [Google Scholar] [CrossRef]
- Enomoto, S.; Sumi, M.; Kajimoto, K.; Nakazawa, Y.; Takahashi, R.; Takabayashi, C.; Asakura, T.; Sata, M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 2010, 51, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Gong, M.-S.; Park, J.-H.; Moon, S.-I.; Wall, I.B.; Kim, H.-W.; Lee, J.H.; Knowles, J.C. Silk fibroin-polyurethane blends: Physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater. 2013, 9, 8962–8971. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, T.; Yu, H.; Zhu, J.; Sun, C.; Wang, J.; Chen, S.; Wang, J.; Guo, X. Potential applications of three-dimensional structure of silk fibroin/poly(ester-urethane) urea nanofibrous scaffold in heart valve tissue engineering. Appl. Surf. Sci. 2018, 447, 269–278. [Google Scholar] [CrossRef]
- Van Uden, S.; Catto, V.; Perotto, G.; Athanassiou, A.; Redaelli, A.C.L.; Greco, F.G.; Riboldi, S.A. Electrospun fibroin/polyurethane hybrid meshes: Manufacturing, characterization, and potentialities as substrates for haemodialysis arteriovenous grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Ibe, Y.; Jono, T.; Naito, A. Structure and dynamics of biodegradable polyurethane-silk fibroin composite materials in the dry and hydrated states studied using 13C solid-state NMR spectroscopy. Polym. Degrad. Stab. 2021, 190, 109645. [Google Scholar] [CrossRef]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef] [Green Version]
- Król, P.; Uram, Ł.; Król, B.; Pielichowska, K.; Sochacka-Piętal, M.; Walczak, M. Synthesis and property of polyurethane elastomer for biomedical applications based on nonaromatic isocyanates, polyesters, and ethylene glycol. Colloid Polym. Sci. 2020, 298, 1077–1093. [Google Scholar] [CrossRef]
- Serrano, M.C.; Pagani, R.; Vallet-Regí, M.; Peña, J.; Rámila, A.; Izquierdo, I.; Portolés, M.T. In vitro biocompatibility assessment of poly(epsilon-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, K.D.; Pechar, T.W.; Hollinger, J.O.; Guelcher, S.A.; Goldstein, A.S. Synthesis and characterization of segmented poly(esterurethane urea) elastomers for bone tissue engineering. Acta Biomater. 2007, 3, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the structure of Bombyx mori silk fibroin by NMR. Macromolecules 2015, 48, 2345–2357. [Google Scholar] [CrossRef]
- Asakura, T.; Endo, M.; Tasei, Y.; Ohkubo, T.; Hiraoki, T. Hydration of Bombyx mori silk cocoon, silk sericin and silk fibroin and their interactions with water as studied by 13C NMR and 2H NMR relaxation. J. Mater. Chem. B 2017, 5, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Endo, M.; Fukuhara, R.; Tasei, Y. 13C NMR characterization of hydrated 13C labeled Bombyx mori silk fibroin sponges prepared using glycerin, poly(ethylene glycol diglycidyl ether) and poly(ethylene glycol) as porogens. J. Mater. Chem. B 2017, 5, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Matsuda, H.; Tasei, Y.; Asakura, T. Effect of Water on the Structure and Dynamics of Regenerated [3-13C] Ser, [3-13C], and [3-13C] Ala-Bombyx mori Silk Fibroin Studied with 13C Solid-State Nuclear Magnetic Resonance. Biomacromolecules 2018, 19, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Uemura, A.; Tanaka, R.; Tasei, Y.; Asakura, T. Comparison of the knitted silk vascular grafts coated with fibroin sponges prepared using glycerin, poly(ethylene glycol diglycidyl ether) and poly(ethylene glycol) as porogens. J. Biomater. Appl. 2018, 32, 1239–1252. [Google Scholar] [CrossRef]
- Pérez-Rigueiro, J.; Madurga, R.; Gañán-Calvo, A.M.; Elices, M.; Guinea, G.V.; Tasei, Y.; Nishimura, A.; Matsuda, H.; Asakura, T. Emergence of supercontraction in regenerated silkworm (Bombyx mori) silk fibers. Sci. Rep. 2019, 9, 2398. [Google Scholar] [CrossRef]
- Asakura, T.; Isobe, K.; Kametani, S.; Ukpebor, O.T.; Silverstein, M.C.; Boutis, G.S. Characterization of water in hydrated Bombyx mori silk fibroin fiber and films by 2H NMR relaxation and 13C solid-state NMR. Acta Biomater. 2017, 50, 322–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakura, T.; Isobe, K.; Aoki, A.; Kametani, S. Conformation of Crystalline and Noncrystalline Domains of [3-13C]Ala-, [3-13C]Ser-, and [3-13C]Tyr-Bombyx mori Silk Fibroin in a Hydrated State Studied with 13C DD/MAS NMR. Macromolecules 2015, 48, 8062–8069. [Google Scholar] [CrossRef]
- Yagi, T.; Sato, M.; Nakazawa, Y.; Tanaka, K.; Sata, M.; Itoh, K.; Takagi, Y.; Asakura, T. Preparation of double-raschel knitted silk vascular grafts and evaluation of short-term function in a rat abdominal aorta. J. Artif. Organs 2011, 14, 89–99. [Google Scholar] [CrossRef]
- Aytemiz, D.; Sakiyama, W.; Suzuki, Y.; Nakaizumi, N.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Nakazawa, Y.; Asakura, T. Small-Diameter Silk Vascular Grafts (3 mm Diameter) with a Double-Raschel Knitted Silk Tube Coated with Silk Fibroin Sponge. Adv. Healthc. Mater. 2013, 2, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yao, J.; Masuda, H.; Raghuvansh, K.; Asakura, T. Structural characterization and artificial fiber formation of Bombyx mori silk fibroin in hexafluoro-iso-propanol solvent system. Biopolymers 2003, 69, 253–259. [Google Scholar] [CrossRef]
- Ha, S.-W.; Asakura, T.; Kishore, R. Distinctive influence of two hexafluoro solvents on the structural stabilization of Bombyx mori silk fibroin protein and its derived peptides: 13C NMR and CD studies. Biomacromolecules 2006, 7, 18–23. [Google Scholar] [CrossRef]
- Saito, H.; Tuzi, S.; Tanio, M.; Naito, A. Dynamic aspects of membrane proteins and membrane-associated peptides as revealed by 13C NMR: Lessons from bacteriorhodopsin as an intact protein. In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 2002; Volume 47, pp. 39–108. [Google Scholar]
- Holland, G.P.; Jenkins, J.E.; Creager, M.S.; Lewis, R.V.; Yarger, J.L. Solid-State NMR Investigation of Major and Minor Ampullate Spider Silk in the Native and Hydrated States. Biomacromolecules 2008, 9, 651–657. [Google Scholar] [CrossRef]
- Kaji, H.; Horii, F. One- and Two-Dimensional Solid-State 13C NMR Analyses of the Solid Structure and Molecular Motion of Poly(ε-caprolactone) Isothermally Crystallized from the Melt. Macromolecules 1997, 30, 5791–5798. [Google Scholar] [CrossRef]
- Schäler, K.; Ostas, E.; Schröter, K.; Thurn-Albrecht, T.; Binder, W.H.; Saalwächter, K. Influence of Chain Topology on Polymer Dynamics and Crystallization. Investigation of Linear and Cyclic Poly(ε-caprolactone)s by 1H Solid-State NMR Methods. Macromolecules 2011, 44, 2743–2754. [Google Scholar] [CrossRef]
- Tonelli, A.E. Calculated γ Effects on the 13C-NMR Spectra of 3,5,7,9,11,13,15-Heptamethylheptadecane Stereoisomers and Their Implications for the Conformational Characteristics of Polypropylene. Macromolecules 1978, 11, 565–567. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Asakura, T. Development of Small-diameter Polyester Vascular Grafts Coated with Silk Fibroin Sponge. Organogenesis 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Sata, M.; Asakura, T. Evaluation of small-diameter silk vascular grafts implanted in dogs. JTCVS Open 2021. [Google Scholar] [CrossRef]
- Liu, H.; Xu, W.; Zou, H.; Ke, G.; Li, W.; Ouyang, C. Feasibility of wet spinning of silk-inspired polyurethane elastic biofiber. Mater. Lett. 2008, 62, 1949–1952. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakazawa, Y.; Derya, A.; Komatsu, T.; Miyazaki, K.; Yamazaki, S.; Asakura, T. Development of silk/polyurethane small-diameter vascular graft by electrospinning. Seikei-Kakou 2013, 25, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.; Mi, H.-Y.; Zhang, J.; Thomson, J.A.; Turng, L.-S. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J. Biomed. Mater. Res. Part A 2018, 106, 985–996. [Google Scholar] [CrossRef]
- Nakazawa, C.T.; Higuchi, A.; Asano, A.; Kameda, T.; Aytemiz, D.; Nakazawa, Y. Solid-state NMR studies for the development of non-woven biomaterials based on silk fibroin and polyurethane. Polym. J. 2017, 49, 583–586. [Google Scholar] [CrossRef]
- Shimada, K.; Higuchi, A.; Kubo, R.; Murakami, T.; Nakazawa, Y.; Tanaka, R. The effect of a silk Fibroin/Polyurethane blend patch on rat Vessels. Organogenesis 2017, 13, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Aytemiz, D.; Fukuda, Y.; Higuchi, A.; Asano, A.; Nakazawa, C.T.; Kameda, T.; Yoshioka, T.; Nakazawa, Y. Compatibility Evaluation of Non-Woven Sheet Composite of Silk Fibroin and Polyurethane in the Wet State. Polymers 2018, 10, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, R.; Konishi, H.; Ozawa, H.; Katsumata, T.; Tanaka, R.; Nakazawa, Y.; Nemoto, S. Development of a new surgical sheet containing both silk fibroin and thermoplastic polyurethane for cardiovascular surgery. Surg. Today 2018, 48, 486–494. [Google Scholar] [CrossRef]
- Riboldi, S.A.; Tozzi, M.; Bagardi, M.; Ravasio, G.; Cigalino, G.; Crippa, L.; Piccolo, S.; Nahal, A.; Spandri, M.; Catto, V.; et al. A Novel Hybrid Silk Fibroin/Polyurethane Arteriovenous Graft for Hemodialysis: Proof-of-Concept Animal Study in an Ovine Model. Adv. Healthc. Mater. 2020, 9, 2000794. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, H.; Schreiber, C.; Grasl, C.; Walter, I.; Plasenzotti, R.; Stoiber, M.; Bernhard, D.; Schima, H. Healing characteristics of electrospun polyurethane grafts with various porosities. Acta Biomater. 2013, 9, 6032–6040. [Google Scholar] [CrossRef]
- Zhang, X.; Baughman, C.B.; Kaplan, D.L. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 2008, 29, 2217–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soffer, L.; Wang, X.; Zhang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Cao, C.; Ma, X. A novel three-dimensional tubular scaffold prepared from silk fibroin by electrospinning. Int. J. Biol. Macromol. 2009, 45, 504–510. [Google Scholar] [CrossRef]
- Marelli, B.; Alessandrino, A.; Farè, S.; Freddi, G.; Mantovani, D.; Tanzi, M.C. Compliant electrospun silk fibroin tubes for small vessel bypass grafting. Acta Biomater. 2010, 6, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Zhou, G.; Fan, H.; Fan, Y. Electrospun sulfated silk fibroin nanofibrous scaffolds for vascular tissue engineering. Biomaterials 2011, 32, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, H.; Huang, C.; Su, Y.; Mo, X.; Ikada, Y. Fabrication of silk fibroin blended P(LLA-CL) nanofibrous scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2010, 93A, 984–993. [Google Scholar] [CrossRef]
- Sato, M.; Nakazawa, Y.; Takahashi, R.; Tanaka, K.; Sata, M.; Aytemiz, D.; Asakura, T. Small-diameter vascular grafts of Bombyx mori silk fibroin prepared by a combination of electrospinning and sponge coating. Mater. Lett. 2010, 64, 1786–1788. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, H.; Dong, Z. Preparation, characterization and biocompatibility of electrospinning heparin-modified silk fibroin nanofibers. Int. J. Biol. Macromol. 2011, 48, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bonani, W.; Maniglio, D.; Motta, A.; Tan, W.; Migliaresi, C. Biohybrid nanofiber constructs with anisotropic biomechanical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96B, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, I.; Figliuzzi, M.; Azzollini, N.; Catto, V.; Farè, S.; Tanzi, M.C.; Alessandrino, A.; Freddi, G.; Remuzzi, A. In vivo regeneration of elastic lamina on fibroin biodegradable vascular scaffold. Int. J. Artif. Organs 2013, 36, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx mori silk by electrospinning—Part 1: Processing parameters and geometric properties. Polymer 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Ohgo, K.; Zhao, C.; Kobayashi, M.; Asakura, T. Preparation of non-woven nanofibers of Bombyx mori silk, Samia cynthia ricini silk and recombinant hybrid silk with electrospinning method. Polymer 2003, 44, 841–846. [Google Scholar] [CrossRef]
- Ercolani, E.; Del Gaudio, C.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2015, 9, 861–888. [Google Scholar] [CrossRef] [PubMed]











| Number of Carbons | CP/MAS | DD/MAS | INEPT |
|---|---|---|---|
| 1 | 65.0 (c) | 63.8 (n) | — |
| 2 | 28.7 (c) | 28.3 (n) | 28.3 (n) |
| 3 | 25.4 | 25.4 | 25.4 |
| 4 | — | 24.5 | 24.5 |
| 5 | 33.0 (c) | 33.7 (n) | 33.7 (n) |
| 6 | 173.2 (c) | 173.5 | — |
| 7 | 21.6 | 21.6 | — |
| 8 | 69.8 | 70.0 | 69.7 |
| 9 | — | 58.2 | 58.3 |
| ACβ | ca.20 (β*),17.1 (rc) | ca.20 (β*),16.8 (rc+hrc) | 16.7 (hrc) |
| ACα | 49.3 (β*),50.0 (rc) | 50.0 (rc+hrc) | 50.0 (hrc) |
| GCα | 42.7 | 42.7 | 42.7 |
| SCβ | —, 61.4 (rc) | —, 61.4 (rc+hrc) | 61.4 (hrc) |
| SCα | 54.3 (β*),55.8(rc) | 55.8(rc+hrc) | 55.8 (hrc) |
| Number of Carbons | CP/MAS | DD/MAS | INEPT |
|---|---|---|---|
| 1 | 64.8 (c) | 63.8 (n) | 63.8 (n) |
| 2 | 28.5 (c) | 28.5 (n) | 28.3 (n) |
| 3 | 25.4 | 25.4 | 25.4 |
| 4 | — | 24.7 | 24.4 |
| 5 | 33.0 (c) | 33.5 (n) | 33.6 (n) |
| 6 | 173.2 (c) | 172.9 | — |
| 7 | 21.6 | 21.6 | 21.5 |
| 8 | 69.7 | 69.8 | 69.7 |
| 9 | — | — | 58.2 |
| ACβ | ca.20 (β*),16.8 (rc) | ca.20 (β*),16.8 (rc+hrc) | 16.7 (hrc) |
| ACα | 49.4 (β*),50.0 (rc) | 50.0 (rc+hrc) | 50.0 (hrc) |
| GCα | 42.7 | 42.7 | 42.7 |
| SCβ | —,61.4 (rc) | —, 61.4 (rc+hrc) | 61.4 (hrc) |
| SCα | 54.3 (β*),55.8(rc) | 55.8(rc+hrc) | 55.8 (hrc) |
| (a) Breaking strength (N) | ||
| n | SF tube without coating | SF tube with coating |
| 1 | 30.5 | 34.4 |
| 2 | 30.3 | 37.9 |
| 3 | 27.9 | 40.4 |
| 4 | 29.1 | 37.5 |
| 5 | 28.3 | 31.9 |
| 6 | 28.2 | 30.8 |
| Average | 29.1 | 35.5 |
| Standard deviation | 1.1 | 3.8 |
| (b) Elongation-at-break (%) | ||
| n | SF tube without coating | SF tube with coating |
| 1 | 154 | 122 |
| 2 | 161 | 114 |
| 3 | 145 | 126 |
| 4 | 129 | 133 |
| 5 | 148 | 107 |
| 6 | 142 | 116 |
| Average | 147 | 120 |
| Standard deviation | 11 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Ibe, Y.; Jono, T.; Tanaka, R.; Naito, A.; Asakura, T. Characterization of a Water-Dispersed Biodegradable Polyurethane-Silk Composite Sponge Using 13C Solid-State Nuclear Magnetic Resonance as Coating Material for Silk Vascular Grafts with Small Diameters. Molecules 2021, 26, 4649. https://doi.org/10.3390/molecules26154649
Tanaka T, Ibe Y, Jono T, Tanaka R, Naito A, Asakura T. Characterization of a Water-Dispersed Biodegradable Polyurethane-Silk Composite Sponge Using 13C Solid-State Nuclear Magnetic Resonance as Coating Material for Silk Vascular Grafts with Small Diameters. Molecules. 2021; 26(15):4649. https://doi.org/10.3390/molecules26154649
Chicago/Turabian StyleTanaka, Takashi, Yusuke Ibe, Takaki Jono, Ryo Tanaka, Akira Naito, and Tetsuo Asakura. 2021. "Characterization of a Water-Dispersed Biodegradable Polyurethane-Silk Composite Sponge Using 13C Solid-State Nuclear Magnetic Resonance as Coating Material for Silk Vascular Grafts with Small Diameters" Molecules 26, no. 15: 4649. https://doi.org/10.3390/molecules26154649