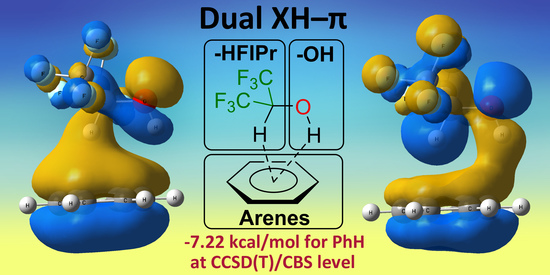
Dual XH–π Interaction of Hexafluoroisopropanol with Arenes
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References and Notes
- Sinha, S.K.; Bhattacharya, T.; Maiti, D. Role of hexafluoroisopropanol in C–H activation. React. Chem. Eng. 2019, 4, 244–253. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Ghosh, A.; Maiti, D. Hexafluoroisopropanol: The magical solvent for Pd-catalyzed C–H activation. Chem. Sci. 2021, 12, 3857–3870. [Google Scholar] [CrossRef]
- Richmond, E.; Vukovic, V.D.; Moran, J. Nucleophilic ring opening of donor-acceptor cyclopropanes catalyzed by a Bronsted acid in hexafluoroisopropanol. Org. Lett. 2018, 20, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Pozhydaiev, V.; Power, M.; Gandon, V.; Moran, J.; Lebœuf, D. Exploiting hexafluoroisopropanol (HFIP) in Lewis and Brønsted acid-catalyzed reactions. Chem. Commun. 2020, 56, 11548–11564. [Google Scholar] [CrossRef] [PubMed]
- Vuković, V.D.; Richmond, E.; Wolf, E.; Moran, J. Catalytic Friedel–Crafts reactions of highly electronically deactivated benzylic alcohols. Angew. Chem. Int. Ed. 2017, 56, 3085–3089. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-X.; Qu, J. Friedel–Crafts alkylation of arenes with epoxides promoted by fluorinated alcohols or water. Chem. Commun. 2010, 46, 2653–2655. [Google Scholar] [CrossRef]
- Champagne, P.A.; Benhassine, Y.; Desroches, J.; Paquin, J.F. Friedel–Crafts reaction of benzyl fluorides: Selective activation of C-F bonds as enabled by hydrogen bonding. Angew. Chem. Int. Ed. 2014, 53, 13835–13839. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, H.F.; Vekariya, R.H.; Aube, J. Intramolecular Friedel–Crafts acylation reaction promoted by 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol. Org. Lett. 2015, 17, 5484–5487. [Google Scholar] [CrossRef]
- Lu, L.; Liu, H.; Hua, R. HNO3/HFIP: A nitrating system for arenes with direct observation of π-complex intermediates. Org. Lett. 2018, 20, 3197–3201. [Google Scholar] [CrossRef]
- Miura, Y.; Satoh, T.; Narumi, A.; Nishizawa, O.; Okamoto, Y.; Kakuchi, T. Synthesis of well-defined syndiotactic poly (methylmethacrylate) with low-temperature atom transfer radical polymerization in fluoroalcohol. J. Polym. Sci. Part Polym. Chem. 2006, 44, 1436–1446. [Google Scholar] [CrossRef]
- Chen, H.; Zong, G.; Chen, L.; Zhang, M.; Wang, C.; Qu, R. Samarium powder as catalyst for SET-LRP of acrylonitrile in 1,1,1,3,3,3-hexafluoro-2-propanol for control of molecular weight and tacticity. J. Polym. Sci. Part Polym. Chem. 2011, 49, 2924–2930. [Google Scholar] [CrossRef]
- Yamada, K.; Nakano, T.; Okamoto, Y. Free-radical copolymerization of vinyl esters using fluoroalcohols as solvents: The sol-vent effect on the monomer reactivity ratio. J. Polym. Sci. Part Polym. Chem. 2000, 38, 220–228. [Google Scholar] [CrossRef]
- Mulla, H.R.; Cammers-Goodwin, A. Stability of a minimalist, aromatic cluster in aqueous mixtures of fluoro alcohol. J. Am. Chem. Soc. 2000, 122, 738–739. [Google Scholar] [CrossRef]
- Waldvogel, S.; Elsler, B. Electrochemical synthesis on boron-doped diamond. Electrochim. Acta. 2012, 82, 434–443. [Google Scholar] [CrossRef]
- Hua, J.; Fang, Z.; Bian, M.; Ma, T.; Yang, M.; Xu, J.; Liu, C.; He, W.; Zhu, N.; Yang, Z. Electrochemical synthesis of spiro [4.5] trienones through radical-initiated dearomative spirocyclization. Chem. Sus. Chem. 2020, 13, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Hollóczki, O.; Berkessel, A.; Mars, J.; Mezger, M.; Wiebe, A.; Waldvogel, S.R.; Kirchner, B. The catalytic effect of fluoro-alcohol mixtures depends on domain formation. ACS Catal. 2017, 7, 1846–1852. [Google Scholar] [CrossRef]
- Hong, D.-P.; Hoshino, M.; Kuboi, R.; Goto, Y. Clustering of fluorine-substituted alcohols as a factor responsible for their marked effects on proteins and peptides. J. Am. Chem. Soc. 1999, 121, 8427–8433. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, T.; Adachi, T.; Otomo, T.; Matsuo, D.; Takamuku, T.; Nishi, N. Structure and dynamics of hex-afluoroisopropanol-water mixtures by x-ray diffraction, small-angle neutron scattering, NMR spectroscopy, and mass spec-trometry. J. Chem. Phys. 2003, 119, 6132–6142. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, S.J.; Sokalski, W.A.; Dyguda, E.; Leszczynski, J. Quantitative classification of covalent and noncovalent H-bonds. J. Phys. Chem. 2006, 110, 6444–6446. [Google Scholar] [CrossRef]
- Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involv-ing carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M.S.; Chakrabarti, P. CH-π hydrogen bonds in biological macromolecules. Phys. Chem. Chem. Phys. 2014, 16, 12648–12683. [Google Scholar] [CrossRef]
- Nakagawa, N. Solvent shifts in NMR spectra of organic compounds. I. The solvation shift of the methyl group. Nippon Kagaku Zassi 1961, 82, 141–147. [Google Scholar] [CrossRef]
- Eberson, L.; Hartshorn, M.P.; Persson, O. 1,1,1,3,3,3-Hexafluoropropan-2-ol as a solvent for the generation of highly persistentradical cations. J. Chem. Soc. Perkin Trans. 1995, 9, 1735–1744. [Google Scholar] [CrossRef]
- Serjeant, E.P.; Dempsey, B. Ionisation constants of organic acids in aqueous solution. IUPAC Chem. Data Ser. 1979, 23, 160–190. [Google Scholar]
- Shahi, A.; Arunan, E. Microwave spectroscopic and theoretical investigations of the strongly hydrogen bonded hexafluoroiso-propanol water complex. Phys. Chem. Chem. Phys. 2015, 17, 24774–24782. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Arunan, E. Microwave spectrum of hexafluoroisopropanol and torsional behavior of molecules with a CF3-C-CF3 group. J. Phys. Chem. 2015, 119, 5650–5657. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, Q.; Cao, W.; Han, J.; Zhou, X.; Liu, S.; Wang, X.B. Probing orientation-specific charge-dipole interactions be-tween hexafluoroisopropanol and halides: A joint photoelectron spectroscopy and theoretical study. J. Phys. Chem. 2020, 124, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functional for main group thermochemistry, thermochemical kinetics, non-covalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Neves, A.R.; Fernandes, P.A.; Ramos, M.J. The accuracy of density functional theory in the description of cation-π and π-hydrogen bond interactions. J. Chem. Theory Comput. 2011, 7, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Legault, C.Y. CYL View, 1.0b, Université de Sherbrooke. 2009. Available online: http://www.cylview.org (accessed on 21 November 2018).
- Berkessel, A.; Adrio, J.A.; Hüttenhain, D.; Neudörfl, J.M. Unveiling the “booster effect” of fluorinated alcohol solvents: Aggre-gation-induced conformational changes and cooperatively enhanced H-bonding. J. Am. Chem. Soc. 2006, 128, 8421–8426. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some pro-cedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M.; Dannenberg, J. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef] [Green Version]
- Fujii, A.; Shibasaki, K.; Kazama, T.; Itaya, R.; Mikami, N.; Tsuzuki, S. Experimental and theoretical determination of the accu-rate interaction energies in benzene–halomethane: The unique nature of the activated CH/π interaction of haloalkanes. Phys. Chem. Chem. Phys. 2008, 10, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Infinite-basis calculations of binding energies for the hydrogen bonded and stacked tetramers of for-mic acid and formamide and their use for validation of hybrid DFT and ab initio methods. J. Phys. Chem. 2005, 109, 6624–6627. [Google Scholar] [CrossRef] [PubMed]
- Fast, P.L.; Sánchez, M.L.; Truhlar, D.G. Infinite basis limits in electronic structure theory. J. Chem. Phys. 1999, 111, 2921–2926. [Google Scholar] [CrossRef]
- Truhlar, D.G. Basis-set extrapolation. Chem. Phys. Lett. 1998, 294, 45–48. [Google Scholar] [CrossRef]
- Jurečka, P.; Hobza, P. On the convergence of the (ΔECCSD (T)-ΔEMP2) term for complexes with multiple H-bonds. Chem. Phys. Lett. 2002, 365, 89–94. [Google Scholar] [CrossRef]
- de Lange, J.H.; van Niekerk, D.M.E.; Cukrowski, I. Quantifying individual (anti)bonding molecular orbitalsʹ contributions to chemical bonding. Phys. Chem. Chem. Phys. 2019, 21, 20988–20998. [Google Scholar] [CrossRef] [PubMed]
- Ruedenberg, K.; Schmidt, M.W. Physical understanding through variational reasoning: Electron sharing and covalent bonding. J. Phys. Chem. 2009, 113, 1954–1968. [Google Scholar] [CrossRef]
- Rocher-Casterline, B.E.; Chʹng, L.C.; Mollner, A.K.; Reisler, H. Communication: Determination of the bond dissociation energy (D0) of the water dimer, (H2O)2, by velocity map imaging. J. Chem. Phys. 2011, 134, 211101. [Google Scholar] [CrossRef] [Green Version]
- Ostojić, B.D.; Janjić, G.V.; Zarić, S.D. Parallel alignment of water and aryl rings—Crystallographic and theoretical evidence for the interaction. Chem. Commun. 2008, 48, 6546–6548. [Google Scholar] [CrossRef]
- Janjić, G.V.; Veljković, D.Z.; Zarić, S.D. Water/aromatic parallel alignment interactions. Significant interactions at large hori-zontal displacements. Cryst. Growth Des. 2011, 11, 2680–2683. [Google Scholar] [CrossRef]
- Veljković, D.Ž.; Janjić, G.V.; Zarić, S.D. Are C–H⋯O interactions linear? The case of aromatic CH donors. Cryst. Eng. Comm. 2011, 13, 5005–5010. [Google Scholar] [CrossRef]
- Meisel, J.W.; Hu, C.T.; Hamilton, A.D. Heterofunctionalized cavitands by macrocyclization of sequence-defined folda-mers. Org. Lett. 2019, 21, 7763–7767. [Google Scholar] [CrossRef]
- The structural data for 1,1,1,3,3,3-hexaflurorisopropyl benzoate (HFIPBz) are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) with reference number CCDC2083861.
- Lu, L.; Hua, R. A monomer-polymer-monomer (MPM) organic synthesis strategy: Synthesis and application of polybenzofuran for functionalizing benzene ring of benzofuran. Asian J. Org. Chem. 2021. [Google Scholar] [CrossRef]





| Method | HFIP/PhH | CHCl3/PhH | IP/PhH |
|---|---|---|---|
| HF/aug-cc-pVDZ | 0.28 | 1.26 | 1.71 |
| HF/aug-cc-pVTZ | 0.26 | 1.21 | 1.66 |
| HF/CBS a | 0.25 | 1.19 | 1.64 |
| MP2/aug-cc-pVDZ | −6.88 | −5.99 | −4.21 |
| MP2/aug-cc-pVTZ | −7.79 | −6.89 | −4.86 |
| MP2/CBS b | −8.17 | −7.27 | −5.13 |
| MP2/6-31+G(d,p) | −5.25 | −3.65 | −2.58 |
| MP2/6-311++G(d,p) | −5.69 | −4.33 | −3.16 |
| CCSD(T)/6-31+G(d,p) | −4.29 | −2.49 | −1.88 |
| CCSD(T)/6-311++G(d,p) | −4.74 | −3.03 | −2.39 |
| Δ(CCSD(T)-MP2) c | 0.96 | 1.16 | 0.70 |
| Δ(CCSD(T)-MP2) d | 0.95 | 1.30 | 0.77 |
| CCSD(T)/CBS e, c | −7.21 | −6.11 | −4.43 |
| CCSD(T)/CBS e, d | −7.22 | −5.97 | −4.36 |
| Ecorrf | −7.47 | −7.16 | −6.00 |
| ΔZPE g | 0.85 | 0.55 | 0.54 |
| M06-2X/6-311++G(2d,2p) h | −7.49 | −5.65 | −4.53 |
| D0 (calculated) i | 6.64 | 5.10 | 3.99 |
| D0 (experimental) | 5.2 ± 0.2 j |
| X-H Donor | ||||
|---|---|---|---|---|
| Entry | X-H Acceptor | HFIP | IP | CHCl3 |
| 1 | ethane | −1.50 | −2.49 | −2.24 |
| 2 | ethylene | −5.31 | −3.23 | −3.27 |
| 3 | acetylene | −5.87 | −3.16 | −2.80 |
| 4 | benzene | −7.49 | −4.53 | −5.65 |
| 5 | HMB | −11.61 | −7.80 | −9.14 |
| 6 | HFB | −2.71 | −4.61 | −2.96 |
| 7 | anisole | −8.60 | −5.51 | −6.63 |
| 8 | naphthalene | −7.71 | −5.79 | −6.25 |
| 9 | cyclohexane | −3.80 | −2.11 | −2.34 |
| 10 | cyclopropane | −5.42 | −3.43 | −2.06 |
| 11 | cubane | −5.26 | −3.64 | −3.89 |
| 12 | H2O | −9.83 | −6.52 | −4.64 |
| 13 | Me2O | −10.45 | −6.20 | −5.41 |
| 14 | Et2O | −12.21 | −7.60 | −6.81 |
| 15 | THF | −11.71 | −7.42 | −6.34 |
| 16 | 1,4-dioxane | −10.38 | −6.46 | −5.21 |
| 17 | ethylene oxide | −9.60 | −6.21 | −5.62 |
| 18 | DMSO | −15.62 | −9.38 | −7.24 |
| 19 | sulfolane | −12.94 | −6.24 | −6.17 |
| 20 | acetone | −11.88 | −7.06 | −6.01 |
| 21 | acetic acid | −11.74 | −5.63 | −6.38 |
| 22 | trimethylamine | −15.09 | −8.41 | −6.50 |
| 23 | acetonitrile | −9.52 | −4.90 | −3.94 |
| 24 | DMAc | −14.88 | −8.83 | −7.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Hua, R. Dual XH–π Interaction of Hexafluoroisopropanol with Arenes. Molecules 2021, 26, 4558. https://doi.org/10.3390/molecules26154558
Lu L, Hua R. Dual XH–π Interaction of Hexafluoroisopropanol with Arenes. Molecules. 2021; 26(15):4558. https://doi.org/10.3390/molecules26154558
Chicago/Turabian StyleLu, Le, and Ruimao Hua. 2021. "Dual XH–π Interaction of Hexafluoroisopropanol with Arenes" Molecules 26, no. 15: 4558. https://doi.org/10.3390/molecules26154558